Inflammatory etiopathogenesis of systemic lupus erythematosus: an update
- PMID: 26316795
- PMCID: PMC4548750
- DOI: 10.2147/JIR.S70325
Inflammatory etiopathogenesis of systemic lupus erythematosus: an update
Abstract
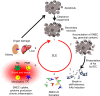
The immune system struggles every day between responding to foreign antigens and tolerating self-antigens to delicately maintain tissue homeostasis. If self-tolerance is broken, the development of autoimmunity can be the consequence, as it is in the case of the chronic inflammatory autoimmune disease systemic lupus erythematosus (SLE). SLE is considered to be a multifactorial disease comprising various processes and cell types that act abnormally and in a harmful way. Oxidative stress, infections, or, in general, tissue injury are accompanied by massive cellular demise. Several processes such as apoptosis, necrosis, or NETosis (formation of Neutrophil Extracellular Traps [NETs]) may occur alone or in combination. If clearance of dead cells is insufficient, cellular debris may accumulate and trigger inflammation and leakage of cytoplasmic and nuclear autoantigens like ribonucleoproteins, DNA, or histones. Inadequate removal of cellular remnants in the germinal centers of secondary lymphoid organs may result in the presentation of autoantigens by follicular dendritic cells to autoreactive B cells that had been generated by chance during the process of somatic hypermutation (loss of peripheral tolerance). The improper exposure of nuclear autoantigens in this delicate location is consequently prone to break self-tolerance to nuclear autoantigens. Indeed, the germline variants of autoantibodies often do not show autoreactivity. The subsequent production of autoantibodies plays a critical role in the development of the complex immunological disorder fostering SLE. Immune complexes composed of cell-derived autoantigens and autoantibodies are formed and get deposited in various tissues, such as the kidney, leading to severe organ damage. Alternatively, they may also be formed in situ by binding to planted antigens of circulating autoantibodies. Here, we review current knowledge about the etiopathogenesis of SLE including the involvement of different types of cell death, serving as the potential source of autoantigens, and impaired clearance of cell remnants, causing accumulation of cellular debris.
Keywords: NETosis; apoptosis; autoimmunity; cell death; clearance deficiency; systemic lupus erythematosus.
Figures

References
-
- Munoz LE, Janko C, Schulze C, et al. Autoimmunity and chronic inflammation – two clearance-related steps in the etiopathogenesis of SLE. Autoimmun Rev. 2010;10(1):38–42. - PubMed
-
- Perniok A, Wedekind F, Herrmann M, Specker C, Schneider M. High levels of circulating early apoptic peripheral blood mononuclear cells in systemic lupus erythematosus. Lupus. 1998;7(2):113–118. - PubMed
-
- Rhodes B, Vyse TJ. The genetics of SLE: an update in the light of genome-wide association studies. Rheumatology (Oxford) 2008;47(11):1603–1611. - PubMed
-
- Edwards CJ. Environmental factors and lupus: are we looking too late? Lupus. 2005;14(6):423–425. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

