The treatment landscape in thyroid cancer: a focus on cabozantinib
- PMID: 26316818
- PMCID: PMC4547654
- DOI: 10.2147/CMAR.S68373
The treatment landscape in thyroid cancer: a focus on cabozantinib
Abstract
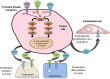
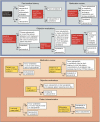
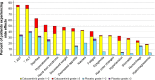
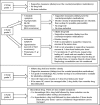
Although patients with thyroid cancer generally fare well, there is a subset for which this is not necessarily true. Progress in understanding the molecular aberrations in thyroid cancer has led to a change in the management of these cases. Since 2011, four multikinase inhibitors (MKIs) have been approved by the US Food and Drug Administration for thyroid cancer - cabozantinib and vandetanib for medullary thyroid cancer and sorafenib and lenvatinib for differentiated thyroid cancer. This change in the treatment landscape has raised challenges for practitioners who may not be familiar with the use of MKIs or with the treatment and natural history of advanced thyroid cancer in general. This article reviews the epidemiology, molecular drivers, and initial treatment of patients with thyroid cancer and offers practical guidance to assist with the determination of when to appropriately start an MKI. As an example, cabozantinib and its efficacy are discussed in detail. Close monitoring is required for all patients on targeted agents to assess for adverse effects and response to therapy. An approach to managing drug-related adverse events is detailed. Since these drugs are not curative and have not yet proven to prolong overall survival, it is critical to weigh the risks and benefits of treatment at every visit. The potential value of changing to a different agent following failure of an MKI is also addressed.
Keywords: RET; VEGF; adverse event; chemotherapy; kinase inhibitor; targeted therapy.
Figures

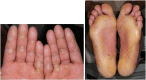
References
-
- COMETRIQ™ [package insert] South San Francisco, CA: Exelixis, Inc.; 2012. [Accessed March 29, 2015]. Available from: http://www.cometriq.com/downloads/Cometriq_Full_Prescribing_Information.pdf.
-
- National Cancer Institute at the National Institutes of Health . Cancer of the Thyroid – SEER Stat Fact Sheets. Bethesda, MD: National Cancer Institute at the National Institutes of Health; [Accessed March 27, 2015]. Available from: http://seer.cancer.gov/statfacts/html/thyro.html.
-
- Ruegemer JJ, Hay ID, Bergstralh EJ, Ryan JJ, Offord KP, Gorman CA. Distant metastases in differentiated thyroid carcinoma: a multivariate analysis of prognostic variables. J Clin Endocrinol Metab. 1988;67(3):501–508. - PubMed
-
- Besic N, Gazic B. Sites of metastases of anaplastic thyroid carcinoma: autopsy findings in 45 cases from a single institution. Thyroid. 2013;23(6):709–713. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

