Predictors of referral for neoadjuvant chemotherapy prior to radical cystectomy for muscle-invasive bladder cancer and changes in practice over time
- PMID: 26316905
- PMCID: PMC4537332
- DOI: 10.5489/cuaj.2722
Predictors of referral for neoadjuvant chemotherapy prior to radical cystectomy for muscle-invasive bladder cancer and changes in practice over time
Abstract
Introduction: In patients with non-metastatic muscle-invasive bladder cancer (MIBC) fit for curative therapy, a multidisciplinary approach consisting is recommended. This approach includes local treatment (usually radical cystectomy), ideally combined with neoadjuvant chemotherapy (NACT). Despite a survival benefit with NACT, uptake remains low. We assessed NACT consultation in Alberta and examined associative factors, as well as the relationship to survival.
Methods: Patients with MIBC were identified through the Alberta Cancer Registry. Demographic and clinicopathologic information was collected from electronic medical records between 2007 and 2011. In addition to descriptive statistics, logistic regression was used to determine factors associated with receiving NACT consultation. Overall survival was described using a Kaplan-Meier estimate.
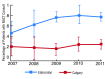
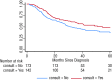
Results: Of the 315 radical cystectomy patients, 140 (45.1%, 95% confidence interval [CI] 39.5, 50.8) received NACT consultation. Patients ≥80 years (odds ratio [OR] 0.21, 95% CI 0.08, 0.57, p = 0.002) and those treated in Calgary (OR 0.11, 95% CI 0.05, 0.25, p < 0.001) were less likely to receive NACT consultation. The rate of NACT consultation increased steadily from 2007 to 2011 (OR 1.23, 95% CI 1.04, 1.45 per year of diagnosis, p = 0.018). After a median follow-up of 28.1 months (range: 14.6-50.3), median survival was 54.7 months for patients who received NACT consultation versus 31.2 months for those who did not (p = 0.030).
Conclusions: NACT consultation in patients with MIBC undergoing radical cystectomy has improved over time; however, regional differences underscore the need for a standardized approach to NACT consultation, including common referral mechanisms.
Figures
References
-
- Statistics 2014. Toronto, ON: Canadian Cancer Society; cancer.ca/statistics. Accessed June 15, 2015.
-
- Statistics Canada CANSIM Tables. http://cansim2.statcan.gc.ca. Accessed June 15, 2015.
-
- Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: A randomized Southwest Oncology Group study. J Urol. 2000;163:1124–9. doi: 10.1016/S0022-5347(05)67707-5. - DOI - PubMed
-
- Taher AN, Kotb MH. Bone metastases in muscle-invasive bladder cancer. J Egypt Natl Canc Inst. 2006;18:203–8. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources