External Quality Assessment for the Detection of Chlamydia trachomatis in Urine Using Molecular Techniques in Belgium
- PMID: 26316982
- PMCID: PMC4496476
- DOI: 10.1155/2015/835261
External Quality Assessment for the Detection of Chlamydia trachomatis in Urine Using Molecular Techniques in Belgium
Abstract
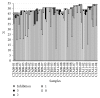
Chlamydia trachomatis is a major cause of sexually transmitted bacterial disease worldwide. C. trachomatis is an intracellular bacterium and its growth in vitro requires cell culture facilities. The diagnosis is based on antigen detection and more recently on molecular nucleic acid amplification techniques (NAAT) that are considered fast, sensitive, and specific. In Belgium, External Quality Assessment (EQA) for the detection of C. trachomatis in urine by NAAT was introduced in 2008. From January 2008 to June 2012, nine surveys were organized. Fifty-eight laboratories participated in at least one survey. The EQA panels included positive and negative samples. The overall accuracy was 75.4%, the overall specificity was 97.6%, and the overall sensitivity was 71.4%. Two major issues were observed: the low sensitivity (45.3%) for the detection of low concentration samples and the incapacity of several methods to detect the Swedish variant of C. trachomatis. The reassuring point was that the overall proficiency of the Belgian laboratories tended to improve over time.
Figures


Similar articles
-
Establishing an External Quality Assessment (EQA) Program for Urinalysis in Medical Laboratories of Thailand.Indian J Clin Biochem. 2024 Apr;39(2):271-275. doi: 10.1007/s12291-022-01102-3. Epub 2022 Nov 28. Indian J Clin Biochem. 2024. PMID: 38577144 Free PMC article.
-
Combination with antimicrobial peptide lyses improves loop-mediated isothermal amplification based method for Chlamydia trachomatis detection directly in urine sample.BMC Infect Dis. 2016 Jul 13;16:329. doi: 10.1186/s12879-016-1674-0. BMC Infect Dis. 2016. PMID: 27412444 Free PMC article.
-
The verification of nucleic acid amplification testing (Gen-Probe Aptima Assay) for chlamydia trachomatis from ocular samples.Ophthalmology. 2015 Feb;122(2):244-7. doi: 10.1016/j.ophtha.2014.08.038. Epub 2014 Oct 14. Ophthalmology. 2015. PMID: 25439605
-
Systematic reviews of point-of-care tests for the diagnosis of urogenital Chlamydia trachomatis infections.Sex Transm Infect. 2017 Dec;93(S4):S22-S30. doi: 10.1136/sextrans-2016-053067. Sex Transm Infect. 2017. PMID: 29223960 Review.
-
DNA amplification assays: a new standard for diagnosis of Chlamydia trachomatis infections.Ann Acad Med Singap. 1995 Jul;24(4):627-33. Ann Acad Med Singap. 1995. PMID: 8849200 Review.
References
-
- Gerbase A. C., Rowley J. T., Heymann D. H. L., Berkley S. F. B., Piot P. Global prevalence and incidence estimates of selected curable STDS. Sexually Transmitted Infections. 1998;74(supplement 1):S12–S16. - PubMed
-
- Ducoffre G. Surveillance des maladies infectieuses par un réseau de laboratoires de microbiologie. Institut Scientifique de Santé Publique; 2010. https://www.wiv-isp.be/epidemio/epifr/plabfr/plabanfr/10_035f_v.pdf.
-
- Gaydos C., Essig A. Chlamydiaceae in Manual of Clinical Microbiology. 10th. Washington, DC, USA: ASM Press; 2011. (edited by: J. Versalovic).
LinkOut - more resources
Full Text Sources
Other Literature Sources

