Neuroprotection by Exendin-4 Is GLP-1 Receptor Specific but DA D3 Receptor Dependent, Causing Altered BrdU Incorporation in Subventricular Zone and Substantia Nigra
- PMID: 26316987
- PMCID: PMC4437329
- DOI: 10.1155/2013/407152
Neuroprotection by Exendin-4 Is GLP-1 Receptor Specific but DA D3 Receptor Dependent, Causing Altered BrdU Incorporation in Subventricular Zone and Substantia Nigra
Abstract
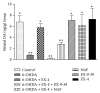
Glucagon-like peptide-1 receptor (GLP-1R) activation by exendin-4 (EX-4) is effective in preclinical models of Parkinson's disease (PD) and appears to promote neurogenesis even in severely lesioned rats. In the present study, we determined the effects of EX-4 on cellular BrdU incorporation in the rat subventricular zone (SVZ) and substantia nigra (SN). We also determined the specificity of this effect with the GLP-1R antagonist EX-(9-39) as well as the potential role of dopamine (DA) D3 receptors. Rats were administered 6-OHDA and 1 week later given EX-4 alone, with EX-(9-39) or nafadotride (D3 antagonist) and BrdU. Seven days later, rats were challenged with apomorphine to evaluate circling. Extracellular DA was measured using striatal microdialysis and subsequently tissue DA measured. Tyrosine hydroxylase and BrdU were verified using immunohistochemistry. Apomorphine circling was reversed by EX-4 in lesioned rats, an effect reduced by EX-4, while both EX-(9-39) and NAF attenuated this. 6-OHDA decreased extracellular and tissue DA, both reversed by EX-4 but again attenuated by EX-(9-39) or NAF. Analysis of BrdU+ cells in the SVZ revealed increases in 6-OHDA-treated rats which were reversed by EX-4 and antagonised by either EX-(9-39) or NAF, while in the SN the opposite profile was seen.
Figures





References
-
- Bertilsson G., Patrone C., Zachrisson O., et al. Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson's disease. Journal of Neuroscience Research. 2008;86(2):326–338. doi: 10.1002/jnr.21483. - DOI - PubMed
-
- Li Y., Perry T., Kindy M. S., et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(4):1285–1290. doi: 10.1073/pnas.0806720106. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

