Bacopa monnieri Phytochemicals Mediated Synthesis of Platinum Nanoparticles and Its Neurorescue Effect on 1-Methyl 4-Phenyl 1,2,3,6 Tetrahydropyridine-Induced Experimental Parkinsonism in Zebrafish
- PMID: 26317003
- PMCID: PMC4437347
- DOI: 10.1155/2013/972391
Bacopa monnieri Phytochemicals Mediated Synthesis of Platinum Nanoparticles and Its Neurorescue Effect on 1-Methyl 4-Phenyl 1,2,3,6 Tetrahydropyridine-Induced Experimental Parkinsonism in Zebrafish
Abstract
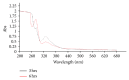
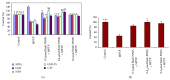
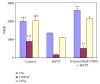
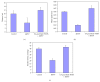
Current discovery demonstrates the rapid formation of platinum nanoparticles using leaf extract of a neurobeneficial plant, Bacopa monnieri (BmE). The nanoparticles (BmE-PtNPs) were stabilized and then coated with varied phytochemicals present within the leaf extract. These nanoparticles demonstrated the same activity of Complex I, as that of oxidizing NADH to NAD(+) using a spectrophotometric method. This suggests that BmE-PtNPs are a potential medicinal substance for oxidative stress mediated disease with suppressed mitochondrial complex I, namely, Parkinson's disease (PD). Hence, the neuroprotective potentials of the phytochemical coated nanoparticle were explored in 1-methyl 4-phenyl 1,2,3,6 tetrahydropyridine- (MPTP-)induced experimental Parkinsonism in zebrafish model. BmE-PtNPs pretreatment significantly reversed toxic effects of MPTP by increasing the levels of dopamine, its metabolites, GSH and activities of GPx, catalase, SOD and complex I, and reducing levels of MDA along with enhanced locomotor activity. Taken together, these findings suggest that BmE-PtNPs have protective effect in MPTP-induced neurotoxicity in this model of Parkinson's disease via their dual functions as mitochondrial complex I and antioxidant activity.
Figures

References
-
- Gerlach M., Riederer P. Animal models of Parkinson's disease: an empirical comparison with the phenomenology of the disease in man. Journal of Neural Transmission. 2006;103(8-9):987–1041. - PubMed
-
- Endo R., Saito T., Asada A., Kawahara H., Ohshima T., Hisanaga S. I. Commitment of 1-methyl-4-phenylpyrinidinium ion-induced neuronal cell deathby proteasome-mediated degradation of p35 cyclin-dependent kinase 5 activator. Journal of Biological Chemistry. 2009;284(38):26029–26039. doi: 10.1074/jbc.M109.026443. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

