A Multiple Antigenic Peptide Mimicking Peptidoglycan Induced T Cell Responses to Protect Mice from Systemic Infection with Staphylococcus aureus
- PMID: 26317210
- PMCID: PMC4552945
- DOI: 10.1371/journal.pone.0136888
A Multiple Antigenic Peptide Mimicking Peptidoglycan Induced T Cell Responses to Protect Mice from Systemic Infection with Staphylococcus aureus
Abstract
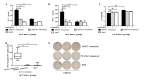
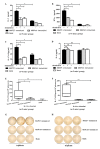
Due to the enormous capacity of Staphylococcus aureus to acquire antibiotic resistance, it becomes imperative to develop vaccines for decreasing the risk of its life-threatening infections. Peptidoglycan (PGN) is a conserved and major component of S. aureus cell wall. However, it has not been used as a vaccine candidate since it is a thymus-independent antigen. In this study, we synthesized a multiple antigenic peptide, named MAP27, which comprised four copies of a peptide that mimics the epitope of PGN. After immunization with MAP27 five times and boosting with heat-inactivated bacterium one time, anti-MAP27 serum bound directly to S. aureus or PGN. Immunization with MAP27 decreased the bacterial burden in organs of BALB/c mice and significantly prolonged their survival time after S. aureus lethal-challenge. The percentage of IFN-γ(+)CD3(+) T cells and IL-17(+)CD4(+) T cells in spleen, as well as the levels of IFN-γ, IL-17A/F and CCL3 in spleen and lung, significantly increased in the MAP27-immunized mice after infection. Moreover, in vitro incubation of heat-inactivated S. aureus with splenocytes isolated from MAP27-immunized mice stimulated the production of IFN-γ and IL-17A/F. Our findings demonstrated that MAP27, as a thymus-dependent antigen, is efficient at eliciting T cell-mediated responses to protect mice from S. aureus infection. This study sheds light on a possible strategy to design vaccines against S. aureus.
Conflict of interest statement
Figures





References
-
- Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998; 339(8): 520–532. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

