14-3-3 Proteins Buffer Intracellular Calcium Sensing Receptors to Constrain Signaling
- PMID: 26317416
- PMCID: PMC4552738
- DOI: 10.1371/journal.pone.0136702
14-3-3 Proteins Buffer Intracellular Calcium Sensing Receptors to Constrain Signaling
Abstract
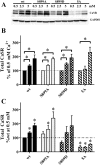
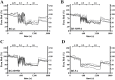
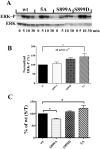
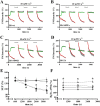
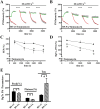
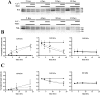
Calcium sensing receptors (CaSR) interact with 14-3-3 binding proteins at a carboxyl terminal arginine-rich motif. Mutations identified in patients with familial hypocalciuric hypercalcemia, autosomal dominant hypocalcemia, pancreatitis or idiopathic epilepsy support the functional importance of this motif. We combined total internal reflection fluorescence microscopy and biochemical approaches to determine the mechanism of 14-3-3 protein regulation of CaSR signaling. Loss of 14-3-3 binding caused increased basal CaSR signaling and plasma membrane levels, and a significantly larger signaling-evoked increase in plasma membrane receptors. Block of core glycosylation with tunicamycin demonstrated that changes in plasma membrane CaSR levels were due to differences in exocytic rate. Western blotting to quantify time-dependent changes in maturation of expressed wt CaSR and a 14-3-3 protein binding-defective mutant demonstrated that signaling increases synthesis to maintain constant levels of the immaturely and maturely glycosylated forms. CaSR thus operates by a feed-forward mechanism, whereby signaling not only induces anterograde trafficking of nascent receptors but also increases biosynthesis to maintain steady state levels of net cellular CaSR. Overall, these studies suggest that 14-3-3 binding at the carboxyl terminus provides an important buffering mechanism to increase the intracellular pool of CaSR available for signaling-evoked trafficking, but attenuates trafficking to control the dynamic range of responses to extracellular calcium.
Conflict of interest statement
Figures

References
-
- Tfelt-Hansen J, Brown E M. The calcium-sensing receptor in normal physiology and pathophysiology: a review. Crit. Rev. Clin. Lab. Sci. 2005; 42: 35–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

