Identification of Novel Short Ragweed Pollen Allergens Using Combined Transcriptomic and Immunoproteomic Approaches
- PMID: 26317427
- PMCID: PMC4552831
- DOI: 10.1371/journal.pone.0136258
Identification of Novel Short Ragweed Pollen Allergens Using Combined Transcriptomic and Immunoproteomic Approaches
Abstract
Background: Allergy to short ragweed (Ambrosia artemisiifolia) pollen is a serious and expanding health problem in North America and Europe. Whereas only 10 short ragweed pollen allergens are officially recorded, patterns of IgE reactivity observed in ragweed allergic patients suggest that other allergens contribute to allergenicity. The objective of the present study was to identify novel allergens following extensive characterization of the transcriptome and proteome of short ragweed pollen.
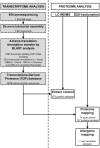
Methods: Following a Proteomics-Informed-by-Transcriptomics approach, a comprehensive transcriptomic data set was built up from RNA-seq analysis of short ragweed pollen. Mass spectrometry-based proteomic analyses and IgE reactivity profiling after high resolution 2D-gel electrophoresis were then combined to identify novel allergens.
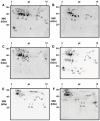
Results: Short ragweed pollen transcripts were assembled after deep RNA sequencing and used to inform proteomic analyses, thus leading to the identification of 573 proteins in the short ragweed pollen. Patterns of IgE reactivity of individual sera from 22 allergic patients were assessed using an aqueous short ragweed pollen extract resolved over 2D-gels. Combined with information derived from the annotated pollen proteome, those analyses revealed the presence of multiple unreported IgE reactive proteins, including new Amb a 1 and Amb a 3 isoallergens as well as 7 novel candidate allergens reacting with IgEs from 20-70% of patients. The latter encompass members of the carbonic anhydrase, enolase, galactose oxidase, GDP dissociation inhibitor, pathogenesis related-17, polygalacturonase and UDP-glucose pyrophosphorylase families.
Conclusions: We extended the list of allergens identified in short ragweed pollen. These findings have implications for both diagnosis and allergen immunotherapy purposes.
Conflict of interest statement
Figures


References
-
- Arbes SJ Jr, Gergen PJ, Elliott L, Zeldin DC (2005) Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol 116: 377–383. - PubMed
-
- D'Amato G, Cecchi L, Bonini S, Nunes C, Annesi-Maesano I, Behrendt H, et al. (2007) Allergenic pollen and pollen allergy in Europe. Allergy 62: 976–990. - PubMed
-
- Asero R, Wopfner N, Gruber P, Gadermaier G, Ferreira F (2006) Artemisia and Ambrosia hypersensitivity: co-sensitization or co-recognition? Clin Exp Allergy 36 658–665. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

