A Distinct Function of Regulatory T Cells in Tissue Protection
- PMID: 26317471
- PMCID: PMC4603556
- DOI: 10.1016/j.cell.2015.08.021
A Distinct Function of Regulatory T Cells in Tissue Protection
Abstract
Regulatory T (Treg) cells suppress immune responses to a broad range of non-microbial and microbial antigens and indirectly limit immune inflammation-inflicted tissue damage by employing multiple mechanisms of suppression. Here, we demonstrate that selective Treg cell deficiency in amphiregulin leads to severe acute lung damage and decreased blood oxygen concentration during influenza virus infection without any measureable alterations in Treg cell suppressor function, antiviral immune responses, or viral load. This tissue repair modality is mobilized in Treg cells in response to inflammatory mediator IL-18 or alarmin IL-33, but not by TCR signaling that is required for suppressor function. These results suggest that, during infectious lung injury, Treg cells have a major direct and non-redundant role in tissue repair and maintenance-distinct from their role in suppression of immune responses and inflammation-and that these two essential Treg cell functions are invoked by separable cues.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
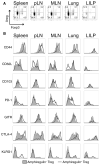
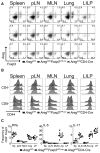


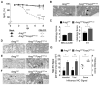

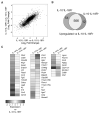
Comment in
-
Regulatory T Cells: Distinct role in tissue repair.Nat Rev Immunol. 2015 Oct;15(10):596-7. doi: 10.1038/nri3915. Epub 2015 Sep 11. Nat Rev Immunol. 2015. PMID: 26358396 No abstract available.
-
The Regulatory T Cell: Jack-Of-All-Trades.Trends Immunol. 2015 Dec;36(12):756-758. doi: 10.1016/j.it.2015.10.002. Epub 2015 Oct 25. Trends Immunol. 2015. PMID: 26511762
References
-
- Berasain C, Avila MA. Amphiregulin. Semin Cell Dev Biol. 2014;28:31–41. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

