Effects of Estrogens on Adipokines and Glucose Homeostasis in Female Aromatase Knockout Mice
- PMID: 26317527
- PMCID: PMC4552801
- DOI: 10.1371/journal.pone.0136143
Effects of Estrogens on Adipokines and Glucose Homeostasis in Female Aromatase Knockout Mice
Abstract
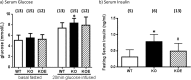
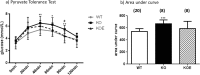
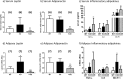
The maintenance of glucose homeostasis within the body is crucial for constant and precise performance of energy balance and is sustained by a number of peripheral organs. Estrogens are known to play a role in the maintenance of glucose homeostasis. Aromatase knockout (ArKO) mice are estrogen-deficient and display symptoms of dysregulated glucose metabolism. We aim to investigate the effects of estrogen ablation and exogenous estrogen administration on glucose homeostasis regulation. Six month-old female wildtype, ArKO, and 17β-estradiol (E2) treated ArKO mice were subjected to whole body tolerance tests, serum examination of estrogen, glucose and insulin, ex-vivo muscle glucose uptake, and insulin signaling pathway analyses. Female ArKO mice display increased body weight, gonadal (omental) adiposity, hyperinsulinemia, and liver triglycerides, which were ameliorated upon estrogen treatment. Tolerance tests revealed that estrogen-deficient ArKO mice were pyruvate intolerant hence reflecting dysregulated hepatic gluconeogenesis. Analyses of skeletal muscle, liver, and adipose tissues supported a hepatic-based glucose dysregulation, with a down-regulation of Akt phosphorylation (a key insulin signaling pathway molecule) in the ArKO liver, which was improved with E2 treatment. Concurrently, estrogen treatment lowered ArKO serum leptin and adiponectin levels and increased inflammatory adipokines such as tumour necrosis factor alpha (TNFα) and interleukin 6 (IL6). Furthermore, estrogen deficiency resulted in the infiltration of CD45 macrophages into gonadal adipose tissues, which cannot be reversed by E2 treatment. This study describes the effects of estrogens on glucose homeostasis in female ArKO mice and highlights a primary phenotype of hepatic glucose dysregulation and a parallel estrogen modified adipokine profile.
Conflict of interest statement
Figures



References
-
- Nordlie RC, Foster JD, Lange AJ. Regulation of glucose production by the liver. Annu Rev Nutr 1999; 19: 379–406. - PubMed
-
- Pilkis SJ, Granner DK. Molecular physiology of the regulation of hepatic gluconeogenesis and glycolysis. Annu Rev Physiol 1992; 54: 885–909. - PubMed
-
- Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001; 414: 799–806. - PubMed
-
- Gabriely I, Ma XH, Yang XM, Atzmon G, Rajala MW, Berg AH et al. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process? Diabetes 2002; 51: 2951–2958. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

