ABT-263 induces apoptosis and synergizes with chemotherapy by targeting stemness pathways in esophageal cancer
- PMID: 26317542
- PMCID: PMC4694873
- DOI: 10.18632/oncotarget.4540
ABT-263 induces apoptosis and synergizes with chemotherapy by targeting stemness pathways in esophageal cancer
Abstract
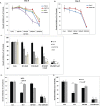
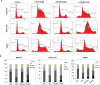
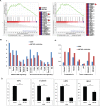
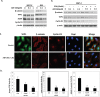
Activation of cancer stem cell signaling is central to acquired resistance to therapy in esophageal cancer (EC). ABT-263, a potent Bcl-2 family inhibitor, is active against many tumor types. However, effect of ABT-263 on EC cells and their resistant counterparts are unknown. Here we report that ABT-263 inhibited cell proliferation and induced apoptosis in human EC cells and their chemo-resistant counterparts. The combination of ABT-263 with 5-FU had synergistic lethal effects and amplified apoptosis that does not depend fully on its inhibition of BCL-2 family proteins in EC cells. To further explore the novel mechanisms of ABT-263, proteomic array (RPPAs) were performed and gene set enriched analysis demonstrated that ABT-263 suppresses the expression of many oncogenes including genes that govern stemness pathways. Immunoblotting and immunofluorescence further confirmed reduction in protein expression and transcription in Wnt/β-catenin and YAP/SOX9 axes. Furthermore, ABT263 strongly suppresses cancer stem cell properties in EC cells and the combination of ABT-263 and 5-FU significantly reduced tumor growth in vivo and suppresses the expression of stemness genes. Thus, our findings demonstrated a novel mechanism of ABT-263 antitumor effect in EC and indicating that combination of ABT-263 with cytotoxic drugs is worthy of pursuit in patients with EC.
Keywords: 5-fluorouracil; ABT-263; cancer stem cells; esophageal cancer; stemness pathways.
Conflict of interest statement
The authors have declared no conflicts of interest.
Figures


Similar articles
-
Metformin sensitizes chemotherapy by targeting cancer stem cells and the mTOR pathway in esophageal cancer.Int J Oncol. 2014 Aug;45(2):567-74. doi: 10.3892/ijo.2014.2450. Epub 2014 May 21. Int J Oncol. 2014. PMID: 24859412 Free PMC article.
-
ABT-263 enhances sorafenib-induced apoptosis associated with Akt activity and the expression of Bax and p21((CIP1/WAF1)) in human cancer cells.Br J Pharmacol. 2014 Jul;171(13):3182-95. doi: 10.1111/bph.12659. Br J Pharmacol. 2014. PMID: 24571452 Free PMC article.
-
Targeting Hippo coactivator YAP1 through BET bromodomain inhibition in esophageal adenocarcinoma.Mol Oncol. 2020 Jun;14(6):1410-1426. doi: 10.1002/1878-0261.12667. Epub 2020 Apr 7. Mol Oncol. 2020. PMID: 32175692 Free PMC article.
-
5-Fluorouracil (5-FU) resistance and the new strategy to enhance the sensitivity against cancer: Implication of DNA repair inhibition.Biomed Pharmacother. 2021 May;137:111285. doi: 10.1016/j.biopha.2021.111285. Epub 2021 Jan 20. Biomed Pharmacother. 2021. PMID: 33485118 Review.
-
Molecular mechanisms associated with chemoresistance in esophageal cancer.Cell Mol Life Sci. 2022 Feb 3;79(2):116. doi: 10.1007/s00018-022-04131-6. Cell Mol Life Sci. 2022. PMID: 35113247 Free PMC article. Review.
Cited by
-
Development of targeted therapy of NRF2high esophageal squamous cell carcinoma.Cell Signal. 2021 Oct;86:110105. doi: 10.1016/j.cellsig.2021.110105. Epub 2021 Aug 4. Cell Signal. 2021. PMID: 34358647 Free PMC article. Review.
-
Senolytic agent ABT-263 mitigates low- and high-LET radiation-induced gastrointestinal cancer development in Apc1638N/+ mice.Aging (Albany NY). 2025 Jan 8;17(1):97-115. doi: 10.18632/aging.206183. Epub 2025 Jan 8. Aging (Albany NY). 2025. PMID: 39792466 Free PMC article.
-
Resistance to Cell Death and Its Modulation in Cancer Stem Cells.Crit Rev Oncog. 2016;21(3-4):203-219. doi: 10.1615/CritRevOncog.2016016976. Crit Rev Oncog. 2016. PMID: 27915972 Free PMC article. Review.
-
Cardiac glycosides are broad-spectrum senolytics.Nat Metab. 2019 Nov;1(11):1074-1088. doi: 10.1038/s42255-019-0122-z. Epub 2019 Oct 21. Nat Metab. 2019. PMID: 31799499 Free PMC article.
-
Potentially Curable Cancers of the Esophagus and Stomach.Mayo Clin Proc. 2016 Sep;91(9):1307-18. doi: 10.1016/j.mayocp.2016.07.018. Mayo Clin Proc. 2016. PMID: 27594190 Free PMC article. Review.
References
-
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64:9–29. - PubMed
-
- van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. The New England journal of medicine. 2012;366:2074–2084. - PubMed
-
- Ajani JA, Barthel JS, Bentrem DJ, D'Amico TA, Das P, Denlinger CS, Fuchs CS, Gerdes H, Glasgow RE, Hayman JA, et al. Esophageal and esophagogastric junction cancers. Journal of the National Comprehensive Cancer Network : JNCCN. 2011;9:830–887. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

