High Platelet Reactivity in Patients with Acute Coronary Syndromes Undergoing Percutaneous Coronary Intervention: Randomised Controlled Trial Comparing Prasugrel and Clopidogrel
- PMID: 26317618
- PMCID: PMC4552627
- DOI: 10.1371/journal.pone.0135037
High Platelet Reactivity in Patients with Acute Coronary Syndromes Undergoing Percutaneous Coronary Intervention: Randomised Controlled Trial Comparing Prasugrel and Clopidogrel
Abstract
Background: Prasugrel is more effective than clopidogrel in reducing platelet aggregation in acute coronary syndromes. Data available on prasugrel reloading in clopidogrel treated patients with high residual platelet reactivity (HRPR) i.e. poor responders, is limited.
Objectives: To determine the effects of prasugrel loading on platelet function in patients on clopidogrel and high platelet reactivity undergoing percutaneous coronary intervention for acute coronary syndrome (ACS).
Patients: Patients with ACS on clopidogrel who were scheduled for PCI found to have a platelet reactivity ≥40 AUC with the Multiplate Analyzer, i.e. "poor responders" were randomised to prasugrel (60 mg loading and 10 mg maintenance dose) or clopidogrel (600 mg reloading and 150 mg maintenance dose). The primary outcome measure was proportion of patients with platelet reactivity <40 AUC 4 hours after loading with study medication, and also at one hour (secondary outcome). 44 patients were enrolled and the study was terminated early as clopidogrel use decreased sharply due to introduction of newer P2Y12 inhibitors.
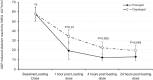
Results: At 4 hours after study medication 100% of patients treated with prasugrel compared to 91% of those treated with clopidogrel had platelet reactivity <40 AUC (p = 0.49), while at 1 hour the proportions were 95% and 64% respectively (p = 0.02). Mean platelet reactivity at 4 and 1 hours after study medication in prasugrel and clopidogrel groups respectively were 12 versus 22 (p = 0.005) and 19 versus 34 (p = 0.01) respectively.
Conclusions: Routine platelet function testing identifies patients with high residual platelet reactivity ("poor responders") on clopidogrel. A strategy of prasugrel rather than clopidogrel reloading results in earlier and more sustained suppression of platelet reactivity. Future trials need to identify if this translates into clinical benefit.
Trial registration: ClinicalTrials.gov NCT01339026.
Conflict of interest statement
Figures


References
-
- Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001–15. - PubMed
-
- Bellemain-Appaix A1, O'Connor SA, Silvain J, Cucherat M, Beygui F, Bartharth O, et al. ACTION Group Association of clopidogrel pretreatment with mortality, cardiovascular events, and major bleeding among patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis. JAMA. 2012;308(23):2507–16. 10.1001/jama.2012.50788 - DOI - PubMed
-
- Ferri 76-87, Sartral M, Tcherny-Lessenot S, Belger M; APTOR trial investigators. A prospective observational study of treatment practice patterns in acute coronary syndrome patients undergoing percutaneous coronary intervention in Europe. Arch Cardiovasc Dis. 2011;104(2):104–14. 10.1016/j.acvd.2010.12.002 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

