Anti-Inflammatory Effects of Lactobacillus Rahmnosus and Bifidobacterium Breve on Cigarette Smoke Activated Human Macrophages
- PMID: 26317628
- PMCID: PMC4552661
- DOI: 10.1371/journal.pone.0136455
Anti-Inflammatory Effects of Lactobacillus Rahmnosus and Bifidobacterium Breve on Cigarette Smoke Activated Human Macrophages
Abstract
Background: Chronic obstructive pulmonary disease (COPD) is a major global health problem with cigarette smoke (CS) as the main risk factor for its development. Airway inflammation in COPD involves the increased expression of inflammatory mediators such as CXCL-8 and IL-1β which are important mediators for neutrophil recruitment. Macrophages are an important source of these mediators in COPD. Lactobacillus rhamnosus (L. rhamnosus) and Befidobacterium breve (B. breve) attenuate the development of 'allergic asthma' in animals but their effects in COPD are unknown.
Objective: To determine the anti-inflammatory effects of L. rhamnosus and B. breve on CS and Toll-like receptor (TLR) activation.
Design: We stimulated the human macrophage cell line THP-1 with CS extract in the presence and absence of L. rhamnosus and B. breve and measured the expression and release of inflammatory mediators by RT-qPCR and ELISA respectively. An activity assay and Western blotting were used to examine NF-κB activation.
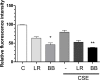
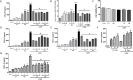
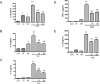
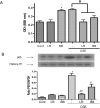
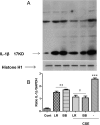
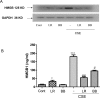
Results: Both L. rhamnosus and B. breve were efficiently phagocytized by human macrophages. L. rhamnosus and B. breve significantly suppressed the ability of CS to induce the expression of IL-1β, IL-6, IL-10, IL-23, TNFα, CXCL-8 and HMGB1 release (all p<0.05) in human THP-1 macrophages. Similar suppression of TLR4- and TLR9-induced CXCL8 expression was also observed (p<0.05). The effect of L. rhamnosus and B. breve on inflammatory mediator release was associated with the suppression of CS-induced NF-κB activation (p<0.05).
Conclusions: This data indicate that these probiotics may be useful anti-inflammatory agents in CS-associated disease such as COPD.
Conflict of interest statement
Figures
References
-
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. American Journal of Respiratory and Critical Care Medicine. 2007;176:532–555. - PubMed
-
- Barnes PJ “Neutrophils find smoke attractive,” Science. 2010; 210:40–41. - PubMed
-
- Rennard SI and Vestbo J COPD: the dangerous underestimate of 15%. The Lancet. 2006;367:1216–1219. - PubMed
-
- Cosio MG, Saetta M, and Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. The New England Journal of Medicine. 2009; 360:2396–2454. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

