Different Blood Cell-Derived Transcriptome Signatures in Cows Exposed to Vaccination Pre- or Postpartum
- PMID: 26317664
- PMCID: PMC4552870
- DOI: 10.1371/journal.pone.0136927
Different Blood Cell-Derived Transcriptome Signatures in Cows Exposed to Vaccination Pre- or Postpartum
Abstract
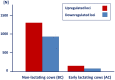
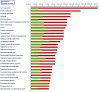
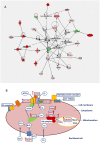
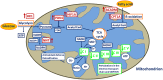
Periparturient cows have been found to reveal immunosuppression, frequently associated with increased susceptibility to uterine and mammary infections. To improve understanding of the causes and molecular regulatory mechanisms accounting for this phenomenon around calving, we examined the effect of an antigen challenge on gene expression modulation on cows prior to (BC) or after calving (AC) using whole transcriptome sequencing (RNAseq). The transcriptome analysis of the cows' blood identified a substantially higher number of loci affected in BC cows (2,235) in response to vaccination compared to AC cows (208) and revealed a divergent transcriptional profile specific for each group. In BC cows, a variety of loci involved in immune defense and cellular signaling processes were transcriptionally activated, whereas protein biosynthesis and posttranslational processes were tremendously impaired in response to vaccination. Furthermore, energy metabolism in the blood cells of BC cows was shifted from oxidative phosphorylation to the glycolytic system. In AC cows, the number and variety of regulated pathways involved in immunomodulation and maintenance of immnunocompetence are considerably lower after vaccination, and upregulation of arginine degradation was suggested as an immunosuppressive mechanism. Elevated transcript levels of erythrocyte-specific genes involved in gas exchange processes were a specific transcriptional signature in AC cows pointing to hematopoiesis activation. The divergent and substantially lower magnitude of transcriptional modulation in response to vaccination in AC cows provides evidence for a suppressed immune capacity of early lactating cows on the molecular level and demonstrates that an efficient immune response of cows is related to their physiological and metabolic status.
Conflict of interest statement
Figures



References
-
- Burvenich C, Bannerman D, Lippolis J, Peelman L, Nonnecke B, Kehrli M, et al. (2007) Cumulative physiological events influence the inflammatory response of the bovine udder to Escherichia coli infections during the transition period. J Dairy Sci 90: E39–E54. - PubMed
-
- Kehrli ME, Harp JA (2001) Immunity in the mammary gland. Vet Clin North Am Food Anim Pract 17: 495–516. - PubMed
-
- Kehrli ME, Nonnecke BJ, Roth JA (1989) Alterations in Bovine Neutrophil Function During the Periparturient Period. Am J Vet Res 50: 207–214. - PubMed
-
- Mallard BA, Dekkers JC, Ireland MJ, Leslie KE, Sharif S, Vankampen CL, et al. (1998) Alteration in immune responsiveness during the peripartum period and its ramification on dairy cow and calf health. J Dairy Sci 81: 585–595. - PubMed
-
- Sheldon IM, Dobson H (2004) Postpartum uterine health in cattle. Anim Reprod Sci 82–3: 295–306. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

