Design, Synthesis, and Cardioprotective Effects of N-Mercapto-Based Hydrogen Sulfide Donors
- PMID: 26317692
- PMCID: PMC4766970
- DOI: 10.1021/acs.jmedchem.5b01033
Design, Synthesis, and Cardioprotective Effects of N-Mercapto-Based Hydrogen Sulfide Donors
Abstract
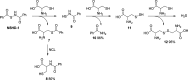
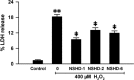
Hydrogen sulfide (H2S) is a signaling molecule which plays regulatory roles in many physiological and/or pathological processes. Therefore, regulation of H2S levels could have great potential therapeutic value. In this work, we report the design, synthesis, and evaluation of a class of N-mercapto (N-SH)-based H2S donors. Thirty-three donors were synthesized and tested. Our results indicated that controllable H2S release from these donors could be achieved upon structural modifications. Selected donors (NSHD-1, NSHD-2, and NSHD-6) were tested in cellular models of oxidative damage and showed significant cytoprotective effects. Moreover, NSHD-1 and NSHD-2 were also found to exhibit potent protective effects in a murine model of myocardial ischemia reperfusion (MI/R) injury.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
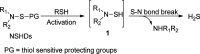








References
-
- Fukuto J. M.; Carrington S. J.; Tantillo D. J.; Harrison J. G.; Ignarro L. J.; Freeman B. A.; Chen A.; Wink D. A. Small molecule signaling agents: the integrated chemistry and biochemistry of nitrogen oxides, oxides of carbon, dioxygen, hydrogen sulfide, and their derived species. Chem. Res. Toxicol. 2012, 25, 769–793. 10.1021/tx2005234. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

