Modulation of Innate Immune Signalling by Lipid-Mediated MAVS Transmembrane Domain Oligomerization
- PMID: 26317833
- PMCID: PMC4552940
- DOI: 10.1371/journal.pone.0136883
Modulation of Innate Immune Signalling by Lipid-Mediated MAVS Transmembrane Domain Oligomerization
Abstract
RIG-I-like receptors detect viral RNA in infected cells and promote oligomerization of the outer mitochondrial membrane protein MAVS to induce innate immunity to viral infection through type I interferon production. Mitochondrial reactive oxygen species (mROS) have been shown to enhance anti-viral MAVS signalling, but the mechanisms have remained obscure. Using a biochemical oligomerization-reporter fused to the transmembrane domain of MAVS, we found that mROS inducers promoted lipid-dependent MAVS transmembrane domain oligomerization in the plane of the outer mitochondrial membrane. These events were mirrored by Sendai virus infection, which similarly induced lipid peroxidation and promoted lipid-dependent MAVS transmembrane domain oligomerization. Our observations point to a role for mROS-induced changes in lipid bilayer properties in modulating antiviral innate signalling by favouring the oligomerization of MAVS transmembrane domain in the outer-mitochondrial membrane.
Conflict of interest statement
Figures


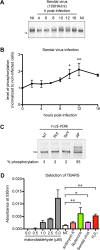
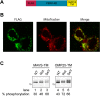
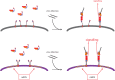
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

