Role of Marine Natural Products in the Genesis of Antiviral Agents
- PMID: 26317854
- PMCID: PMC4883660
- DOI: 10.1021/cr4006318
Role of Marine Natural Products in the Genesis of Antiviral Agents
Abstract
Conflict of interest statement
The authors declare no competing financial interest.
Figures
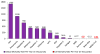
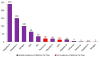
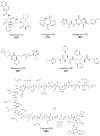
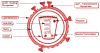

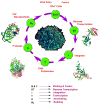
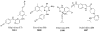
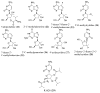
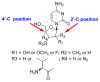
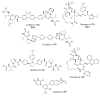
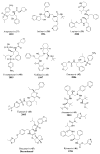
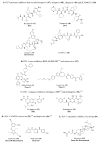
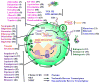
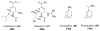
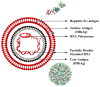
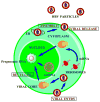
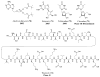

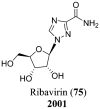

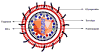
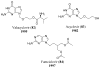
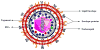


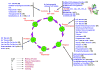
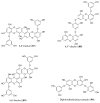
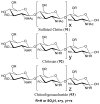
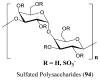
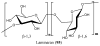
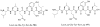
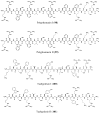
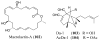
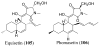
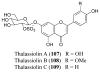
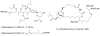
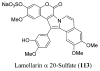
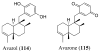

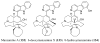
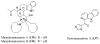
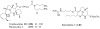
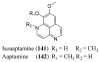
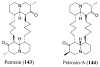
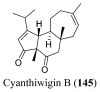
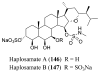
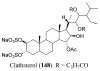
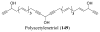
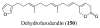

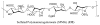
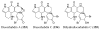
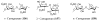
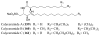
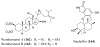
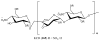
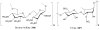

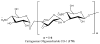

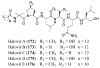
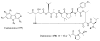
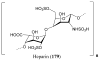
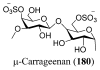
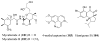
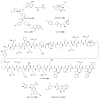
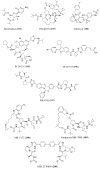
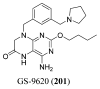
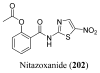
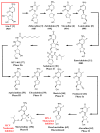
References
-
- MedicineNet.com. [accessed November 25, 2011];Definition of Agent, Anti-Infective. http://www.medicinenet.com/script/main/art.asp?articlekey=2178.
-
- World Health Organization. [accessed May 15, 2014];Media Centre: Fact Sheets. http://www.who.int/mediacentre/factsheets/en/#A.
-
- National Institute of Allergy and Infectious Diseases. [accessed May 15, 2014];Health & Research Topics. http://www.niaid.nih.gov/topics/Pages/default.aspx.
-
- Centers for Disease Control and Prevention. [accessed May 15, 2014];Morbidity and Mortality Weekly Report (MMWR) http://www.cdc.gov/mmwr/indss_2014.html.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

