Three Dimensional Culture of Human Renal Cell Carcinoma Organoids
- PMID: 26317980
- PMCID: PMC4552551
- DOI: 10.1371/journal.pone.0136758
Three Dimensional Culture of Human Renal Cell Carcinoma Organoids
Abstract
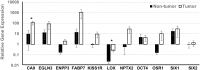
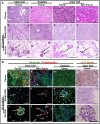
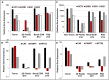
Renal cell carcinomas arise from the nephron but are heterogeneous in disease biology, clinical behavior, prognosis, and response to systemic therapy. Development of patient-specific in vitro models that efficiently and faithfully reproduce the in vivo phenotype may provide a means to develop personalized therapies for this diverse carcinoma. Studies to maintain and model tumor phenotypes in vitro were conducted with emerging three-dimensional culture techniques and natural scaffolding materials. Human renal cell carcinomas were individually characterized by histology, immunohistochemistry, and quantitative PCR to establish the characteristics of each tumor. Isolated cells were cultured on renal extracellular matrix and compared to a novel polysaccharide scaffold to assess cell-scaffold interactions, development of organoids, and maintenance of gene expression signatures over time in culture. Renal cell carcinomas cultured on renal extracellular matrix repopulated tubules or vessel lumens in renal pyramids and medullary rays, but cells were not observed in glomeruli or outer cortical regions of the scaffold. In the polysaccharide scaffold, renal cell carcinomas formed aggregates that were loosely attached to the scaffold or free-floating within the matrix. Molecular analysis of cell-scaffold constructs including immunohistochemistry and quantitative PCR demonstrated that individual tumor phenotypes could be sustained for up to 21 days in culture on both scaffolds, and in comparison to outcomes in two-dimensional monolayer cultures. The use of three-dimensional scaffolds to engineer a personalized in vitro renal cell carcinoma model provides opportunities to advance understanding of this disease.
Conflict of interest statement
Figures

References
-
- NIH National Cancer Institute SEER Stat Fact Sheets: Kidney and Renal Pelvis Cancer. http://www.cancer.gov/researchandfunding/snapshots/kidney.
-
- Ebert T, Bander NH, Finstad CL, Ramsawak RD, Old LJ. Establishment and characterization of human renal cancer and normal kidney cell lines. Cancer Res. 1990;50: 5531–5536. - PubMed
-
- Anglard P, Trahan E, Liu S, Latif F, Merino MJ, Lerman MI, et al. Molecular and cellular characterization of human renal cell carcinoma cell lines. Cancer Res. 1992;52: 348–356. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

