Inhibition of mTORC1 Enhances the Translation of Chikungunya Proteins via the Activation of the MnK/eIF4E Pathway
- PMID: 26317997
- PMCID: PMC4552638
- DOI: 10.1371/journal.ppat.1005091
Inhibition of mTORC1 Enhances the Translation of Chikungunya Proteins via the Activation of the MnK/eIF4E Pathway
Abstract
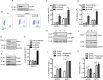
Chikungunya virus (CHIKV), the causative agent of a major epidemic spanning five continents, is a positive stranded mRNA virus that replicates using the cell's cap-dependent translation machinery. Despite viral infection inhibiting mTOR, a metabolic sensor controls cap-dependent translation, viral proteins are efficiently translated. Rapalog treatment, silencing of mtor or raptor genes, but not rictor, further enhanced CHIKV infection in culture cells. Using biochemical assays and real time imaging, we demonstrate that this effect is independent of autophagy or type I interferon production. Providing in vivo evidence for the relevance of our findings, mice treated with mTORC1 inhibitors exhibited increased lethality and showed a higher sensitivity to CHIKV. A systematic evaluation of the viral life cycle indicated that inhibition of mTORC1 has a specific positive effect on viral proteins, enhancing viral replication by increasing the translation of both structural and nonstructural proteins. Molecular analysis defined a role for phosphatidylinositol-3 kinase (PI3K) and MAP kinase-activated protein kinase (MnKs) activation, leading to the hyper-phosphorylation of eIF4E. Finally, we demonstrated that in the context of CHIKV inhibition of mTORC1, viral replication is prioritized over host translation via a similar mechanism. Our study reveals an unexpected bypass pathway by which CHIKV protein translation overcomes viral induced mTORC1 inhibition.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- The 2005–2007 Chikungunya epidemic in Réunion: ambiguous etiologies, memories, and meaning-making. (2013) The 2005–2007 Chikungunya epidemic in Réunion: ambiguous etiologies, memories, and meaning-making. 32: 174–189. Available: http://www.tandfonline.com/doi/abs/10.1080/01459740.2012.679981. 10.1080/01459740.2012.679981 - DOI - PubMed
-
- Chikungunya outbreak in bueng kan province, Thailand, 2013. (2014) Chikungunya outbreak in bueng kan province, Thailand, 2013. 20: 1404–1406. Available: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id.... 10.3201/eid2008.140481 - DOI - PMC - PubMed
-
- Re-emergence of chikungunya virus. (2014) Re-emergence of chikungunya virus.: JVI.01432–14. Available: http://jvi.asm.org/cgi/doi/10.1128/JVI.01432-14. - DOI - PMC - PubMed
-
- Notes from the field: chikungunya virus spreads in the Americas—Caribbean and South America, 2013–2014. (2014) Notes from the field: chikungunya virus spreads in the Americas—Caribbean and South America, 2013–2014. 63: 500–501. Available: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id.... - PMC - PubMed
-
- Proteomic analysis of chikungunya virus infected microgial cells. (2012) Proteomic analysis of chikungunya virus infected microgial cells. 7: e34800 Available: http://dx.plos.org/10.1371/journal.pone.0034800. 10.1371/journal.pone.0034800 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

