Comparative Genomic, MicroRNA, and Tissue Analyses Reveal Subtle Differences between Non-Diabetic and Diabetic Foot Skin
- PMID: 26318001
- PMCID: PMC4552836
- DOI: 10.1371/journal.pone.0137133
Comparative Genomic, MicroRNA, and Tissue Analyses Reveal Subtle Differences between Non-Diabetic and Diabetic Foot Skin
Abstract
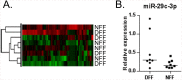
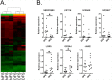
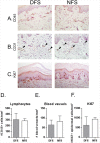
Diabetes Mellitus (DM) is a chronic, severe disease rapidly increasing in incidence and prevalence and is associated with numerous complications. Patients with DM are at high risk of developing diabetic foot ulcers (DFU) that often lead to lower limb amputations, long term disability, and a shortened lifespan. Despite this, the effects of DM on human foot skin biology are largely unknown. Thus, the focus of this study was to determine whether DM changes foot skin biology predisposing it for healing impairment and development of DFU. Foot skin samples were collected from 20 patients receiving corrective foot surgery and, using a combination of multiple molecular and cellular approaches, we performed comparative analyses of non-ulcerated non-neuropathic diabetic foot skin (DFS) and healthy non-diabetic foot skin (NFS). MicroRNA (miR) profiling of laser captured epidermis and primary dermal fibroblasts from both DFS and NFS samples identified 5 miRs de-regulated in the epidermis of DFS though none reached statistical significance. MiR-31-5p and miR-31-3p were most profoundly induced. Although none were significantly regulated in diabetic fibroblasts, miR-29c-3p showed a trend of up-regulation, which was confirmed by qPCR in a prospective set of 20 skin samples. Gene expression profiling of full thickness biopsies identified 36 de-regulated genes in DFS (>2 fold-change, unadjusted p-value ≤ 0.05). Of this group, three out of seven tested genes were confirmed by qPCR: SERPINB3 was up-regulated whereas OR2A4 and LGR5 were down-regulated in DFS. However no morphological differences in histology, collagen deposition, and number of blood vessels or lymphocytes were found. No difference in proliferative capacity was observed by quantification of Ki67 positive cells in epidermis. These findings suggest DM causes only subtle changes to foot skin. Since morphology, mRNA and miR levels were not affected in a major way, additional factors, such as neuropathy, vascular complications, or duration of DM, may further compromise tissue's healing ability leading to development of DFUs.
Conflict of interest statement
Figures




References
-
- Perez MI, Kohn SR. Cutaneous manifestations of diabetes mellitus. Journal of the American Academy of Dermatology. 1994;30(4):519–31; quiz 32–4. . - PubMed
-
- Van Hattem S, Bootsma AH, Thio HB. Skin manifestations of diabetes. Cleveland Clinic journal of medicine. 2008;75(11):772, 4,, 6–7 passim. . - PubMed
-
- Reiber GE, Boyko E.J., and Smith D.G. Lower extremity foot ulcers and amputations in diabetes In: Stern MIHaMP, editor. Diabetes in America. Bethesda, Maryland, USA.: U. S. Government Printing Office; 1995. p. 409–28.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

