The etiology of human age-related cataract. Proteins don't last forever
- PMID: 26318017
- PMCID: PMC5489112
- DOI: 10.1016/j.bbagen.2015.08.016
The etiology of human age-related cataract. Proteins don't last forever
Abstract
Background: It is probable that the great majority of human cataract results from the spontaneous decomposition of long-lived macromolecules in the human lens. Breakdown/reaction of long-lived proteins is of primary importance and recent proteomic analysis has enabled the identification of the particular crystallins, and their exact sites of amino acid modification.
Scope of review: Analysis of proteins from cataractous lenses revealed that there are sites on some structural proteins that show a consistently greater degree of deterioration than age-matched normal lenses.
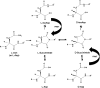
Major conclusions: The most abundant posttranslational modification of aged lens proteins is racemization. Deamidation, truncation and crosslinking, each arising from the spontaneous breakdown of susceptible amino acids within proteins, are also present. Fundamental to an understanding of nuclear cataract etiology, it is proposed that once a certain degree of modification at key sites occurs, that protein-protein interactions are disrupted and lens opacification ensues.
General significance: Since long-lived proteins are now recognized to be present in many other sites of the body, such as the brain, the information gleaned from detailed analyses of degraded proteins from aged lenses will apply more widely to other age-related human diseases. This article is part of a Special Issue entitled Crystallin Biochemistry in Health and Disease.
Keywords: Crystallins; Human aging; Lens; Lifespan; Protein degradation.
Copyright © 2015 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
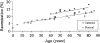

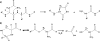
References
-
- Harding J. Cataract biochemistry, Epidemiology and Pharmacology. Chapman and Hall; London: 1991.
-
- Giblin FJ, McCready JP, Reddy VN. The role of glutathione metabolism in the detoxification of H2O2 in rabbit lens. Investigative Ophthalmology & Visual Science. 1982;22:330–335. - PubMed
-
- McFall-Ngai MJ, Ding LL, Takemoto LJ, Horwitz J. Spatial and temporal mapping of the age-related changes in human lens crystallins. Experimental Eye Research. 1985;41:745–758. - PubMed
-
- Truscott RJW. Age-related nuclear cataract—oxidation is the key. Experimental Eye Research. 2005;80:709–725. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

