A Designed Metalloenzyme Achieving the Catalytic Rate of a Native Enzyme
- PMID: 26318313
- PMCID: PMC4676421
- DOI: 10.1021/jacs.5b07119
A Designed Metalloenzyme Achieving the Catalytic Rate of a Native Enzyme
Abstract
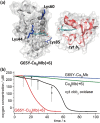
Terminal oxidases catalyze four-electron reduction of oxygen to water, and the energy harvested is utilized to drive the synthesis of adenosine triphosphate. While much effort has been made to design a catalyst mimicking the function of terminal oxidases, most biomimetic catalysts have much lower activity than native oxidases. Herein we report a designed oxidase in myoglobin with an O2 reduction rate (52 s(-1)) comparable to that of a native cytochrome (cyt) cbb3 oxidase (50 s(-1)) under identical conditions. We achieved this goal by engineering more favorable electrostatic interactions between a functional oxidase model designed in sperm whale myoglobin and its native redox partner, cyt b5, resulting in a 400-fold electron transfer (ET) rate enhancement. Achieving high activity equivalent to that of native enzymes in a designed metalloenzyme offers deeper insight into the roles of tunable processes such as ET in oxidase activity and enzymatic function and may extend into applications such as more efficient oxygen reduction reaction catalysts for biofuel cells.
Figures


Similar articles
-
Mobile cytochrome c2 and membrane-anchored cytochrome cy are both efficient electron donors to the cbb3- and aa3-type cytochrome c oxidases during respiratory growth of Rhodobacter sphaeroides.J Bacteriol. 2001 Mar;183(6):2013-24. doi: 10.1128/JB.183.6.2013-2024.2001. J Bacteriol. 2001. PMID: 11222600 Free PMC article.
-
[Applicability of molecular electrostatic interaction models to describing ionic strength dependence of reaction rate between myoglobin and cytochrome c].Biofizika. 1998 Jan-Feb;43(1):16-25. Biofizika. 1998. PMID: 9567172 Russian.
-
Enzyme-catalyzed O2 removal system for electrochemical analysis under ambient air: application in an amperometric nitrate biosensor.Anal Chem. 2012 Mar 6;84(5):2141-6. doi: 10.1021/ac2020883. Epub 2012 Feb 10. Anal Chem. 2012. PMID: 22263529
-
Emerging strategies for expanding the toolbox of enzymes in biocatalysis.Curr Opin Chem Biol. 2020 Apr;55:45-51. doi: 10.1016/j.cbpa.2019.12.006. Epub 2020 Jan 11. Curr Opin Chem Biol. 2020. PMID: 31935627 Free PMC article. Review.
-
Designer metalloenzymes for synthetic biology: Enzyme hybrids for catalysis.Curr Opin Chem Biol. 2020 Oct;58:63-71. doi: 10.1016/j.cbpa.2020.06.004. Epub 2020 Aug 5. Curr Opin Chem Biol. 2020. PMID: 32768658 Review.
Cited by
-
Thioester synthesis by a designed nickel enzyme models prebiotic energy conversion.Proc Natl Acad Sci U S A. 2022 Jul 26;119(30):e2123022119. doi: 10.1073/pnas.2123022119. Epub 2022 Jul 18. Proc Natl Acad Sci U S A. 2022. PMID: 35858422 Free PMC article.
-
Spectroscopic and Crystallographic Evidence for the Role of a Water-Containing H-Bond Network in Oxidase Activity of an Engineered Myoglobin.J Am Chem Soc. 2016 Feb 3;138(4):1134-7. doi: 10.1021/jacs.5b12004. Epub 2016 Jan 20. J Am Chem Soc. 2016. PMID: 26716352 Free PMC article.
-
Insights Into How Heme Reduction Potentials Modulate Enzymatic Activities of a Myoglobin-based Functional Oxidase.Angew Chem Int Ed Engl. 2017 Jun 1;56(23):6622-6626. doi: 10.1002/anie.201701916. Epub 2017 May 4. Angew Chem Int Ed Engl. 2017. PMID: 28470988 Free PMC article.
-
An Engineered Glutamate in Biosynthetic Models of Heme-Copper Oxidases Drives Complete Product Selectivity by Tuning the Hydrogen-Bonding Network.Biochemistry. 2021 Feb 2;60(4):346-355. doi: 10.1021/acs.biochem.0c00852. Epub 2021 Jan 19. Biochemistry. 2021. PMID: 33464878 Free PMC article.
-
Design of Heteronuclear Metalloenzymes.Methods Enzymol. 2016;580:501-37. doi: 10.1016/bs.mie.2016.05.050. Epub 2016 Jul 26. Methods Enzymol. 2016. PMID: 27586347 Free PMC article.
References
-
- Reedy C. J.; Gibney B. R. Chem. Rev. 2004, 104, 617–65010.1021/cr0206115. - DOI - PubMed
- Ueno T.; Abe S.; Yokoi N.; Watanabe Y. Coord. Chem. Rev. 2007, 251, 2717–273110.1016/j.ccr.2007.04.007. - DOI
- Das R.; Baker D. Annu. Rev. Biochem. 2008, 77, 363–38210.1146/annurev.biochem.77.062906.171838. - DOI - PubMed
- Lu Y.; Yeung N.; Sieracki N.; Marshall N. M. Nature 2009, 460, 855.10.1038/nature08304. - DOI - PMC - PubMed
- Heinisch T.; Ward T. R. Curr. Opin. Chem. Biol. 2010, 14, 184–19910.1016/j.cbpa.2009.11.026. - DOI - PubMed
- Kiss G.; Çelebi-Ölçüm N.; Moretti R.; Baker D.; Houk K. N. Angew. Chem., Int. Ed. 2013, 52, 5700–572510.1002/anie.201204077. - DOI - PubMed
- Zastrow M. L.; Pecoraro V. L. Coord. Chem. Rev. 2013, 257, 2565–258810.1016/j.ccr.2013.02.007. - DOI - PMC - PubMed
- Dürrenberger M.; Ward T. R. Curr. Opin. Chem. Biol. 2014, 19, 99–10610.1016/j.cbpa.2014.01.018. - DOI - PubMed
- Petrik I. D.; Liu J.; Lu Y. Curr. Opin. Chem. Biol. 2014, 19, 67–7510.1016/j.cbpa.2014.01.006. - DOI - PMC - PubMed
- Zastrow M. L.; Peacock A. F.; Stuckey J. A.; Pecoraro V. L. Nat. Chem. 2012, 4, 118–12310.1038/nchem.1201. - DOI - PMC - PubMed
- Joh N. H.; Wang T.; Bhate M. P.; Acharya R.; Wu Y.; Grabe M.; Hong M.; Grigoryan G.; DeGrado W. F. Science 2014, 346, 1520–152410.1126/science.1261172. - DOI - PMC - PubMed
- Kleingardner J. G.; Kandemir B.; Bren K. L. J. Am. Chem. Soc. 2014, 136, 4–710.1021/ja406818h. - DOI - PubMed
- Bachmeier A.; Armstrong F. Curr. Opin. Chem. Biol. 2015, 25, 141–15110.1016/j.cbpa.2015.01.001. - DOI - PubMed
- Ray K.; Heims F.; Schwalbe M.; Nam W. Curr. Opin. Chem. Biol. 2015, 25, 159–17110.1016/j.cbpa.2015.01.014. - DOI - PubMed
-
- Fabian M.; Skultety L.; Jancura D.; Palmer G. Biochim. Biophys. Acta, Bioenerg. 2004, 1655, 298–30510.1016/j.bbabio.2003.07.008. - DOI - PubMed
- Brzezinski P.; Gennis R. B. J. Bioenerg. Biomembr. 2008, 40, 521–3110.1007/s10863-008-9181-7. - DOI - PMC - PubMed
- Wikstrom M. Biochim. Biophys. Acta, Bioenerg. 2012, 1817, 468–7510.1016/j.bbabio.2011.10.010. - DOI - PubMed
- Yoshikawa S.; Shimada A. Chem. Rev. 2015, 115, 1936–198910.1021/cr500266a. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

