A Designed Metalloenzyme Achieving the Catalytic Rate of a Native Enzyme
- PMID: 26318313
- PMCID: PMC4676421
- DOI: 10.1021/jacs.5b07119
A Designed Metalloenzyme Achieving the Catalytic Rate of a Native Enzyme
Abstract
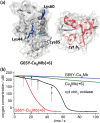
Terminal oxidases catalyze four-electron reduction of oxygen to water, and the energy harvested is utilized to drive the synthesis of adenosine triphosphate. While much effort has been made to design a catalyst mimicking the function of terminal oxidases, most biomimetic catalysts have much lower activity than native oxidases. Herein we report a designed oxidase in myoglobin with an O2 reduction rate (52 s(-1)) comparable to that of a native cytochrome (cyt) cbb3 oxidase (50 s(-1)) under identical conditions. We achieved this goal by engineering more favorable electrostatic interactions between a functional oxidase model designed in sperm whale myoglobin and its native redox partner, cyt b5, resulting in a 400-fold electron transfer (ET) rate enhancement. Achieving high activity equivalent to that of native enzymes in a designed metalloenzyme offers deeper insight into the roles of tunable processes such as ET in oxidase activity and enzymatic function and may extend into applications such as more efficient oxygen reduction reaction catalysts for biofuel cells.
Figures


References
-
- Reedy C. J.; Gibney B. R. Chem. Rev. 2004, 104, 617–650 10.1021/cr0206115. - DOI - PubMed
- Ueno T.; Abe S.; Yokoi N.; Watanabe Y. Coord. Chem. Rev. 2007, 251, 2717–2731 10.1016/j.ccr.2007.04.007. - DOI
- Das R.; Baker D. Annu. Rev. Biochem. 2008, 77, 363–382 10.1146/annurev.biochem.77.062906.171838. - DOI - PubMed
- Lu Y.; Yeung N.; Sieracki N.; Marshall N. M. Nature 2009, 460, 855. 10.1038/nature08304. - DOI - PMC - PubMed
- Heinisch T.; Ward T. R. Curr. Opin. Chem. Biol. 2010, 14, 184–199 10.1016/j.cbpa.2009.11.026. - DOI - PubMed
- Kiss G.; Çelebi-Ölçüm N.; Moretti R.; Baker D.; Houk K. N. Angew. Chem., Int. Ed. 2013, 52, 5700–5725 10.1002/anie.201204077. - DOI - PubMed
- Zastrow M. L.; Pecoraro V. L. Coord. Chem. Rev. 2013, 257, 2565–2588 10.1016/j.ccr.2013.02.007. - DOI - PMC - PubMed
- Dürrenberger M.; Ward T. R. Curr. Opin. Chem. Biol. 2014, 19, 99–106 10.1016/j.cbpa.2014.01.018. - DOI - PubMed
- Petrik I. D.; Liu J.; Lu Y. Curr. Opin. Chem. Biol. 2014, 19, 67–75 10.1016/j.cbpa.2014.01.006. - DOI - PMC - PubMed
- Zastrow M. L.; Peacock A. F.; Stuckey J. A.; Pecoraro V. L. Nat. Chem. 2012, 4, 118–123 10.1038/nchem.1201. - DOI - PMC - PubMed
- Joh N. H.; Wang T.; Bhate M. P.; Acharya R.; Wu Y.; Grabe M.; Hong M.; Grigoryan G.; DeGrado W. F. Science 2014, 346, 1520–1524 10.1126/science.1261172. - DOI - PMC - PubMed
- Kleingardner J. G.; Kandemir B.; Bren K. L. J. Am. Chem. Soc. 2014, 136, 4–7 10.1021/ja406818h. - DOI - PubMed
- Bachmeier A.; Armstrong F. Curr. Opin. Chem. Biol. 2015, 25, 141–151 10.1016/j.cbpa.2015.01.001. - DOI - PubMed
- Ray K.; Heims F.; Schwalbe M.; Nam W. Curr. Opin. Chem. Biol. 2015, 25, 159–171 10.1016/j.cbpa.2015.01.014. - DOI - PubMed
-
- Fabian M.; Skultety L.; Jancura D.; Palmer G. Biochim. Biophys. Acta, Bioenerg. 2004, 1655, 298–305 10.1016/j.bbabio.2003.07.008. - DOI - PubMed
- Brzezinski P.; Gennis R. B. J. Bioenerg. Biomembr. 2008, 40, 521–31 10.1007/s10863-008-9181-7. - DOI - PMC - PubMed
- Wikstrom M. Biochim. Biophys. Acta, Bioenerg. 2012, 1817, 468–75 10.1016/j.bbabio.2011.10.010. - DOI - PubMed
- Yoshikawa S.; Shimada A. Chem. Rev. 2015, 115, 1936–1989 10.1021/cr500266a. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

