Tumor Necrosis Factor-α (TNFα)-induced Ceramide Generation via Ceramide Synthases Regulates Loss of Focal Adhesion Kinase (FAK) and Programmed Cell Death
- PMID: 26318452
- PMCID: PMC4646185
- DOI: 10.1074/jbc.M115.658658
Tumor Necrosis Factor-α (TNFα)-induced Ceramide Generation via Ceramide Synthases Regulates Loss of Focal Adhesion Kinase (FAK) and Programmed Cell Death
Abstract
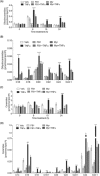
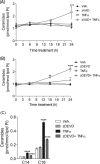
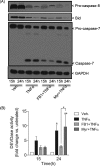
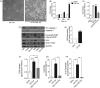
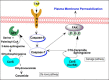
Ceramide synthases (CerS1-CerS6), which catalyze the N-acylation of the (dihydro)sphingosine backbone to produce (dihydro)ceramide in both the de novo and the salvage or recycling pathway of ceramide generation, have been implicated in the control of programmed cell death. However, the regulation of the de novo pathway compared with the salvage pathway is not fully understood. In the current study, we have found that late accumulation of multiple ceramide and dihydroceramide species in MCF-7 cells treated with TNFα occurred by up-regulation of both pathways of ceramide synthesis. Nevertheless, fumonisin B1 but not myriocin was able to protect from TNFα-induced cell death, suggesting that ceramide synthase activity is crucial for the progression of cell death and that the pool of ceramide involved derives from the salvage pathway rather than de novo biosynthesis. Furthermore, compared with control cells, TNFα-treated cells exhibited reduced focal adhesion kinase and subsequent plasma membrane permeabilization, which was blocked exclusively by fumonisin B1. In addition, exogenously added C6-ceramide mimicked the effects of TNFα that lead to cell death, which were inhibited by fumonisin B1. Knockdown of individual ceramide synthases identified CerS6 and its product C16-ceramide as the ceramide synthase isoform essential for the regulation of cell death. In summary, our data suggest a novel role for CerS6/C16-ceramide as an upstream effector of the loss of focal adhesion protein and plasma membrane permeabilization, via the activation of caspase-7, and identify the salvage pathway as the critical mechanism of ceramide generation that controls cell death.
Keywords: Ceramide; PTK2 protein tyrosine kinase 2 (PTK2) (focal adhesion kinase) (FAK); cell death; ceramide synthase; fumonisin b1; plasma membrane; sphingolipid.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures






References
-
- Fulda S. (2009) Tumor resistance to apoptosis. Int. J. Cancer 124, 511–515 - PubMed
-
- Wyllie A. H., Kerr J. F., Currie A. R. (1980) Cell death: the significance of apoptosis. Int. Rev. Cytol. 68, 251–306 - PubMed
-
- Silva M. T. (2010) Secondary necrosis: the natural outcome of the complete apoptotic program. FEBS Lett. 584, 4491–4499 - PubMed
-
- Robertson A. M., Bird C. C., Waddell A. W., Currie A. R. (1978) Morphological aspects of glucocorticoid-induced cell death in human lymphoblastoid cells. J. Pathol. 126, 181–187 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

