Plasmodium Apicoplast Gln-tRNAGln Biosynthesis Utilizes a Unique GatAB Amidotransferase Essential for Erythrocytic Stage Parasites
- PMID: 26318454
- PMCID: PMC4705961
- DOI: 10.1074/jbc.M115.655100
Plasmodium Apicoplast Gln-tRNAGln Biosynthesis Utilizes a Unique GatAB Amidotransferase Essential for Erythrocytic Stage Parasites
Abstract
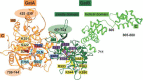
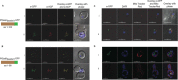
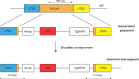
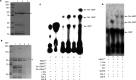
The malaria parasite Plasmodium falciparum apicoplast indirect aminoacylation pathway utilizes a non-discriminating glutamyl-tRNA synthetase to synthesize Glu-tRNA(Gln) and a glutaminyl-tRNA amidotransferase to convert Glu-tRNA(Gln) to Gln-tRNA(Gln). Here, we show that Plasmodium falciparum and other apicomplexans possess a unique heterodimeric glutamyl-tRNA amidotransferase consisting of GatA and GatB subunits (GatAB). We localized the P. falciparum GatA and GatB subunits to the apicoplast in blood stage parasites and demonstrated that recombinant GatAB converts Glu-tRNA(Gln) to Gln-tRNA(Gln) in vitro. We demonstrate that the apicoplast GatAB-catalyzed reaction is essential to the parasite blood stages because we could not delete the Plasmodium berghei gene encoding GatA in blood stage parasites in vivo. A phylogenetic analysis placed the split between Plasmodium GatB, archaeal GatE, and bacterial GatB prior to the phylogenetic divide between bacteria and archaea. Moreover, Plasmodium GatA also appears to have emerged prior to the bacterial-archaeal phylogenetic divide. Thus, although GatAB is found in Plasmodium, it emerged prior to the phylogenetic separation of archaea and bacteria.
Keywords: apicoplast; glutamyl-tRNA amidotransferase; glutamyl-tRNA synthetase; malaria; nucleic acid; nucleic acid enzymology; plasmodium; transfer RNA (tRNA).
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- World Health Organization (2014) World Malaria Report 2014. World Health Organization, Geneva, Switzerland
-
- Goodman C. D., and McFadden G. I. (2013) Targeting apicoplasts in malaria parasites. Expert Opin. Ther. Targets 17, 167–177 - PubMed
-
- Wilson R. J., Denny P. W., Preiser P. R., Rangachari K., Roberts K., Roy A., Whyte A., Strath M., Moore D. J., Moore P. W., and Williamson D. H. (1996) Complete gene map of the plastid-like DNA of the malaria parasite Plasmodium falciparum. J. Mol. Biol. 261, 155–172 - PubMed
-
- Gardner M. J., Feagin J. E., Moore D. J., Spencer D. F., Gray M. W., Williamson D. H., and Wilson R. J. (1991) Organisation and expression of small subunit ribosomal RNA genes encoded by a 35-kilobase circular DNA in Plasmodium falciparum. Mol. Biochem. Parasitol. 48, 77–88 - PubMed
-
- Gardner M. J., Williamson D. H., and Wilson R. J. (1991) A circular DNA in malaria parasites encodes an RNA polymerase like that of prokaryotes and chloroplasts. Mol. Biochem. Parasitol. 44, 115–123 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

