Prenatal stress programs neuroendocrine stress responses and affective behaviors in second generation rats in a sex-dependent manner
- PMID: 26318631
- PMCID: PMC4642655
- DOI: 10.1016/j.psyneuen.2015.08.010
Prenatal stress programs neuroendocrine stress responses and affective behaviors in second generation rats in a sex-dependent manner
Abstract
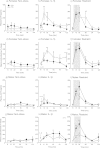
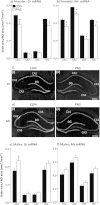
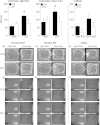
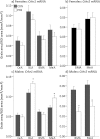
An adverse environment in early life is often associated with dysregulation of the hypothalamo-pituitary-adrenal (HPA) axis and higher rates of mood disorders in adulthood. In rats, exposure to social stress during pregnancy results in hyperactive HPA axis responses to stress in the adult offspring and heightened anxiety behavior in the males, but not the females. Here we tested whether, without further intervention, the effects of prenatal stress (PNS) in the first filial generation (F1) are transmitted to the F2 generation via the maternal line. F1 control and PNS female rats were mated with control males and housed under non-stress conditions throughout pregnancy. HPA axis responses to acute stress, anxiety- and depressive-like behavior were assessed in the adult F2 offspring. ACTH and corticosterone responses to an acute stressor were markedly enhanced in F2 PNS females compared with controls. This was associated with greater corticotropin releasing hormone (Crh) mRNA expression in the paraventricular nucleus and reduced hippocampal glucocorticoid (Gr) and mineralocorticoid receptor (Mr) mRNA expression. Conversely, in the F2 PNS males, HPA axis responses to acute stress were attenuated and hippocampal Gr mRNA expression was greater compared with controls. F2 PNS males exhibited heightened anxiety-like behavior (light-dark box and elevated plus maze) compared with F2 control males. Anxiety-like behavior did not differ between F2 control and PNS females during metestrus/diestrus, however at proestrus/estrus, F2 control females displayed a reduction in anxiety-like behavior, but this effect was not observed in the F2 PNS females. Heightened anxiety in the F2 PNS males was associated with greater Crh mRNA expression in the central nucleus of the amygdala compared with controls. Moreover, Crh receptor-1 (Crhr1) mRNA expression was significantly increased, whereas Crhr2 mRNA was significantly decreased in discrete regions of the amygdala in F2 PNS males compared with controls, with no differences in the F2 females. No differences in depressive-like behavior (sucrose preference or forced swim test) were observed in either sex. In conclusion, the effects of maternal stress during pregnancy on HPA axis regulation and anxiety-like behavior can be transmitted to future generations in a sex-dependent manner. These data have implications for human neuropsychiatric disorders with developmental origins.
Keywords: Anxiety; Glucocorticoids; HPA axis; Prenatal stress; Sex differences; Trans-generational.
Copyright © 2015 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- Abe H., Hidaka N., Kawagoe C., Odagiri K., Watanabe Y., Ikeda T., Ishizuka Y., Hashiguchi H., Takeda R., Nishimori T., Ishida Y. Prenatal psychological stress causes higher emotionality, depression-like behavior, and elevated activity in the hypothalamo-pituitary-adrenal axis. Neurosci. Res. 2007;59:145–151. - PubMed
-
- Bale T.L., Contarino A., Smith G.W., Chan R., Gold L.H., Sawchenko P.E., Koob G.F., Vale W.W., Lee K.F. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat. Genet. 2000;24:410–414. - PubMed
-
- Bale T.L., Vale W.W. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu. Rev. Pharmacol. Toxicol. 2004;44:525–557. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

