Dendritic and Axonal Architecture of Individual Pyramidal Neurons across Layers of Adult Human Neocortex
- PMID: 26318661
- PMCID: PMC4635923
- DOI: 10.1093/cercor/bhv188
Dendritic and Axonal Architecture of Individual Pyramidal Neurons across Layers of Adult Human Neocortex
Abstract
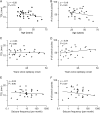
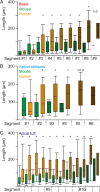
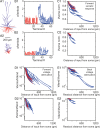
The size and shape of dendrites and axons are strong determinants of neuronal information processing. Our knowledge on neuronal structure and function is primarily based on brains of laboratory animals. Whether it translates to human is not known since quantitative data on "full" human neuronal morphologies are lacking. Here, we obtained human brain tissue during resection surgery and reconstructed basal and apical dendrites and axons of individual neurons across all cortical layers in temporal cortex (Brodmann area 21). Importantly, morphologies did not correlate to etiology, disease severity, or disease duration. Next, we show that human L(ayer) 2 and L3 pyramidal neurons have 3-fold larger dendritic length and increased branch complexity with longer segments compared with temporal cortex neurons from macaque and mouse. Unsupervised cluster analysis classified 88% of human L2 and L3 neurons into human-specific clusters distinct from mouse and macaque neurons. Computational modeling of passive electrical properties to assess the functional impact of large dendrites indicates stronger signal attenuation of electrical inputs compared with mouse. We thus provide a quantitative analysis of "full" human neuron morphologies and present direct evidence that human neurons are not "scaled-up" versions of rodent or macaque neurons, but have unique structural and functional properties.
© The Author 2015. Published by Oxford University Press.
Figures





References
-
- Aarts E, Verhage M, Veenvliet JV, Dolan CV, van der Sluis S. 2014. A solution to dependency: using multilevel analysis to accommodate nested data. Nat Neurosci. 17:491–496. - PubMed
-
- Anderson K, Bones B, Robinson B, Hass C, Lee H, Ford K, Roberts TA, Jacobs B. 2009. The morphology of supragranular pyramidal neurons in the human insular cortex: a quantitative Golgi study. Cereb Cortex. 19:2131–2144. - PubMed
-
- Arimatsu Y, Ishida M, Kaneko T, Ichinose S, Omori A. 2003. Organization and development of corticocortical associative neurons expressing the orphan nuclear receptor Nurr1. J Comp Neurol. 466:180–196. - PubMed
-
- Ascoli GA. 2006. Mobilizing the base of neuroscience data: the case of neuronal morphologies. Nat Rev Neurosci. 7:318–324. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

