Lectin-based analysis of fucosylated glycoproteins of human skim milk during 47 days of lactation
- PMID: 26318738
- PMCID: PMC4651984
- DOI: 10.1007/s10719-015-9615-5
Lectin-based analysis of fucosylated glycoproteins of human skim milk during 47 days of lactation
Abstract
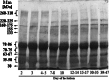
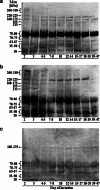
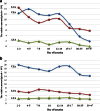
Glycoproteins of human milk are multifunctional molecules, and their fucosylated variants are potentially active molecules in immunological events ensuring breastfed infants optimal development and protection against infection diseases. The expression of fucosylated glycotopes may correspond to milk maturation stages. The relative amounts of fucosylated glycotopes of human skim milk glycoproteins over the course of lactation from the 2(nd) day to the 47(th) day were analyzed in colostrums, transitional and mature milk samples of 43 healthy mothers by lectin-blotting using α1-2-, α1-6-, and α1-3-fucose specific biotinylated Ulex europaeus (UEA), Lens culinaris (LCA), and Lotus tetragonolobus (LTA) lectins, respectively. The reactivities of UEA and LCA with the milk glycoproteins showed the highest expression of α1-2- and α1-6-fucosylated glycotopes on colostrum glycoproteins. The level of UEA-reactive glycoproteins from the beginning of lactation to the 14(th) day was high and relatively stable in contrast to LCA-reactive glycoproteins, the level of which significantly decreased from 2-3 to 7-8 days then remained almost unchanged until the 12(th)-14(th) days. Next, during the progression of lactation the reactivities with both lectins declined significantly. Eighty percent of α1-2- and/or α1-6-fucosylated glycoproteins showed a high negative correlation with milk maturation. In contrast, most of the analyzed milk glycoproteins were not recognized or weakly recognized by LTA and remained at a low unchanged level over lactation. Only a 30-kDa milk glycoprotein was evidently LTA-reactive, showing a negative correlation with milk maturation. The gradual decline of high expression of α1-2- and α1-6-, but not α1-3-, fucoses on human milk glycoproteins of healthy mothers over lactation was associated with milk maturation.
Keywords: Fucose; Fucosylated glycotopes; Human milk glycoproteins; Lactation; Lectins.
Figures
References
-
- ESPGHAN Committee on Nutrition. Agostoni C, Braegger C, Decsi T, Kolacek S, Koletzko B, Michaelsen KF, Mihatsch W, Moreno LA, Puntis J, Shamir R, Szajewska H, Turck D, van Goudoever J. Breast-feeding: a commentary by the ESPGHAN committee on nutrition. J. Pediatr. Gastroenterol. Nutr. 2009;49:112–125. doi: 10.1097/MPG.0b013e31819f1e05. - DOI - PubMed
-
- Ruiz-Palacios GM, Cervantes LE, Ramos P, Chavez-Munguia B, Newburg DS. Campylobacter jejuni binds intestinal H(O) antigen (Fuc alpha 1, 2Gal beta 1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J. Biol. Chem. 2003;278:14112–14120. doi: 10.1074/jbc.M207744200. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

