Concentration-dependent rheological properties of ECM hydrogel for intracerebral delivery to a stroke cavity
- PMID: 26318805
- PMCID: PMC4609617
- DOI: 10.1016/j.actbio.2015.08.040
Concentration-dependent rheological properties of ECM hydrogel for intracerebral delivery to a stroke cavity
Abstract
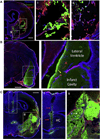
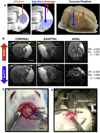
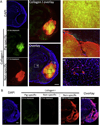
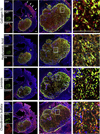
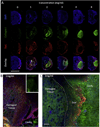
Biomaterials composed of mammalian extracellular matrix (ECM) promote constructive tissue remodeling with minimal scar tissue formation in many anatomical sites. However, the optimal shape and form of ECM scaffold for each clinical application can vary markedly. ECM hydrogels have been shown to promote chemotaxis and differentiation of neuronal stem cells, but minimally invasive delivery of such scaffold materials to the central nervous system (CNS) would require an injectable form. These ECM materials can be manufactured to exist in fluid phase at room temperature, while forming hydrogels at body temperature in a concentration-dependent fashion. Implantation into the lesion cavity after a stroke could hence provide a means to support endogenous repair mechanisms. Herein, we characterize the rheological properties of an ECM hydrogel composed of urinary bladder matrix (UBM) that influence its delivery and in vivo interaction with host tissue. There was a notable concentration-dependence in viscosity, stiffness, and elasticity; all characteristics important for minimally invasive intracerebral delivery. An efficient MRI-guided injection with drainage of fluid from the cavity is described to assess in situ hydrogel formation and ECM retention at different concentrations (0, 1, 2, 3, 4, and 8mg/mL). Only ECM concentrations >3mg/mL gelled within the stroke cavity. Lower concentrations were not retained within the cavity, but extensive permeation of the liquid phase ECM into the peri-infarct area was evident. The concentration of ECM hydrogel is hence an important factor affecting gelation, host-biomaterial interface, as well intra-lesion distribution.
Statement of significance: Extracellular matrix (ECM) hydrogel promotes constructive tissue remodeling in many tissues. Minimally invasive delivery of such scaffold materials to the central nervous system (CNS) would require an injectable form that exists in fluid phase at room temperature, while forming hydrogels at body temperature in a concentration-dependent fashion. We here report the rheological characterization of an injectable ECM hydrogel and its concentration-dependent delivery into a lesion cavity formed after a stroke based on MRI-guidance. The concentration of ECM determined its retention within the cavity or permeation into tissue and hence influenced its interaction with the host brain. This study demonstrates the importance of understanding the structure-function relationship of biomaterials to guide particular clinical applications.
Keywords: Biomaterial; Brain; Delivery; Extracellular matrix; Injection; Magnetic resonance imaging; Stereotactic; Stroke.
Copyright © 2015 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures



References
-
- Modo M, Ambrosio F, Friedlander RM, Badylak SF, Wechsler LR. Bioengineering solutions for neural repair and recovery in stroke. Curr. Opin. Neurol. 2013;26:626–631. - PubMed
-
- Ashioti M, Beech JS, Lowe AS, Hesselink MB, Modo M, Williams SC. Multimodal characterisation of the neocortical clip model of focal cerebral ischaemia by MRI, behaviour and immunohistochemistry. Brain Res. 2007;1145:177–189. - PubMed
-
- Kondziolka D, Steinberg GK, Cullen SB, McGrogan M. Evaluation of surgical techniques for neuronal cell transplantation used in patients with stroke. Cell Transplant. 2004;13:749–754. - PubMed
-
- Meng F, Modo M, Badylak SF. Biologic scaffold for CNS repair. Regen. Med. 2014;9:367–383. - PubMed
-
- Cheng TY, Chen MH, Chang WH, Huang MY, Wang TW. Neural stem cells encapsulated in a functionalized self-assembling peptide hydrogel for brain tissue engineering. Biomaterials. 2013;34:2005–2016. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

