Colchicine in cardiac disease: a systematic review and meta-analysis of randomized controlled trials
- PMID: 26318871
- PMCID: PMC4553011
- DOI: 10.1186/s12872-015-0068-3
Colchicine in cardiac disease: a systematic review and meta-analysis of randomized controlled trials
Abstract
Background: Colchicine has unique anti-inflammatory properties that may be beneficial in various cardiovascular conditions. This systematic review and meta-analysis of randomized controlled trials (RCTs) examines this issue.
Methods: We searched MEDLINE, EMBASE, and the Cochrane Database from inception to June 2014 for RCTs using colchicine in adult patients with cardiac diseases. Results were pooled using random effects.
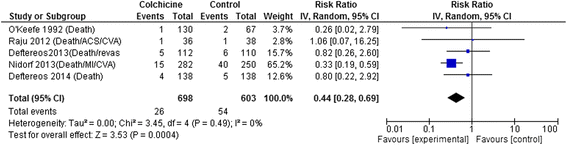
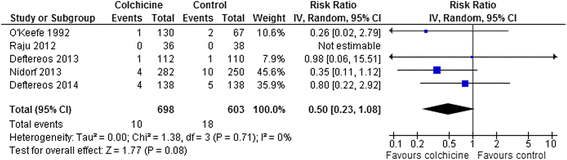
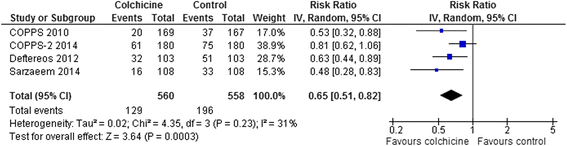
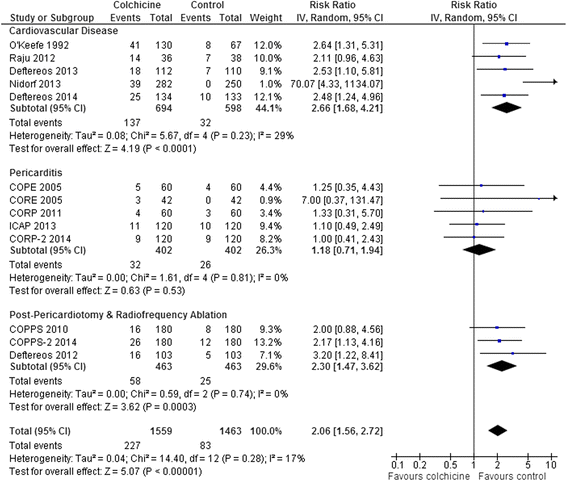
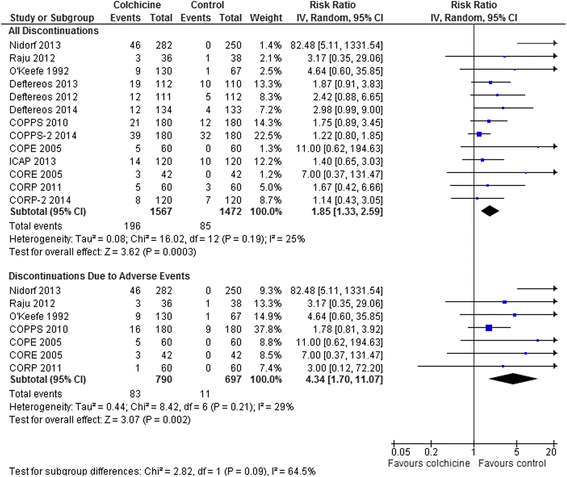
Results: 15 RCTs (n = 3431 patients, median treatment 3 and follow-up 15 months) were included. All but 2 used colchicine 1 mg/day. In 5 trials, n = 1301) at risk for cardiovascular disease (coronary artery disease, acute coronary syndrome or stroke, post-angioplasty [2 RCTs], or congestive heart failure), colchicine reduced composite cardiovascular outcomes by ~60 % (risk ratio [RR] 0.44, 95 % confidence interval [CI] 0.28-0.69, p = 0.0004; I(2) = 0 %) and showed a trend towards lower all-cause mortality (RR 0.50, 95 % CI 0.23-1.08, p = 0.08; I(2) = 0 %). In pericarditis or post-cardiotomy, colchicine decreased recurrent pericarditis or post-pericardiotomy syndrome (RR 0.50, 95 % CI 0.41-0.60, p < 0.0001; I(2) = 0 %; 8 RCTs, n = 1635), and post-pericardiotomy or ablation induced atrial fibrillation (RR 0.65, 95 % CI 0.51-0.82, p = 0.0003; I(2) = 31 %; 4 RCTs, n = 1118). The most common adverse event was diarrhea. Treatment discontinuation overall and due to adverse events (RR 4.34, 95 % CI 1.70-11.07, p = 0.002; I(2) = 29 %; 7 RCTs, 83/790 [10.5 %] vs. 11/697 [1.6 %]) was higher in colchicine-assigned patients.
Conclusions: Current RCT data suggests that colchicine may reduce the composite rate of cardiovascular adverse outcomes in a range of patients with established cardiovascular disease. Furthermore, colchicine reduces rates of recurrent pericarditis, post-pericardiotomy syndrome, and peri-procedural atrial fibrillation following cardiac surgery. Further RCTs evaluating the potential of colchicine for secondary prevention of cardiovascular events would be of interest.
Figures


References
-
- Terkeltaub RA, Furst DE, Bennett K, Kook KA, Crockett RS, Davis MW. High versus low dosing of oral colchicine for early acute gout flare: Twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis Rheum. 2010;62:1060–1068. doi: 10.1002/art.27327. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

