Post-HTS case report and structural alert: Promiscuous 4-aroyl-1,5-disubstituted-3-hydroxy-2H-pyrrol-2-one actives verified by ALARM NMR
- PMID: 26318992
- PMCID: PMC6002837
- DOI: 10.1016/j.bmcl.2015.08.020
Post-HTS case report and structural alert: Promiscuous 4-aroyl-1,5-disubstituted-3-hydroxy-2H-pyrrol-2-one actives verified by ALARM NMR
Abstract
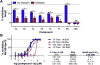
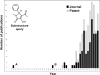
Despite its wide use, not every high-throughput screen (HTS) yields chemical matter suitable for drug development campaigns, and seldom are 'go/no-go' decisions in drug discovery described in detail. This case report describes the follow-up of a 4-aroyl-1,5-disubstituted-3-hydroxy-2H-pyrrol-2-one active from a cell-free HTS to identify small-molecule inhibitors of Rtt109-catalyzed histone acetylation. While this compound and structural analogs inhibited Rtt109-catalyzed histone acetylation in vitro, further work on this series was halted after several risk mitigation strategies were performed. Compounds with this chemotype had a poor structure-activity relationship, exhibited poor selectivity among other histone acetyltransferases, and tested positive in a β-lactamase counter-screen for chemical aggregates. Furthermore, ALARM NMR demonstrated compounds with this chemotype grossly perturbed the conformation of the La protein. In retrospect, this chemotype was flagged as a 'frequent hitter' in an analysis of a large corporate screening deck, yet similar compounds have been published as screening actives or chemical probes versus unrelated biological targets. This report-including the decision-making process behind the 'no-go' decision-should be informative for groups engaged in post-HTS triage and highlight the importance of considering physicochemical properties in early drug discovery.
Keywords: Chemical aggregation; Drug discovery; High-throughput screening; PAINS; Pan-assay interference compounds; Structure–activity relationships.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures



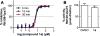
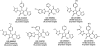
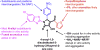
References
-
- Wipf P, Arnold D, Carter K, Dong S, Johnston PA, Sharlow E, Lazo JS, Huryn D. Curr Top Med Chem. 2009;9:1194. - PubMed
-
- Devine S, Mulcair M, Debono C, Leung E, Nissink J, Lim S, Chandrashekaran I, Vazirani M, Mohanty B, Simpson J, Baell J, Scammells P, Norton R, Scanlon M. J Med Chem. 2015;58:1205. - PubMed
-
- Han J, Zhou H, Horazdovsky B, Zhang K, Xu R, Zhang Z. Science. 2007;315:653. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

