Towards detection and diagnosis of Ebola virus disease at point-of-care
- PMID: 26319169
- PMCID: PMC4601610
- DOI: 10.1016/j.bios.2015.08.040
Towards detection and diagnosis of Ebola virus disease at point-of-care
Abstract
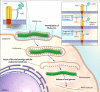
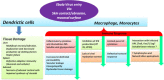
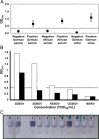
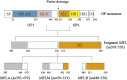
Ebola outbreak-2014 (mainly Zaire strain related Ebola virus) has been declared most widely spread deadly persistent epidemic due to unavailability of rapid diagnostic, detection, and therapeutics. Ebola virus disease (EVD), a severe viral hemorrhagic fever syndrome caused by Ebola virus (EBOV) is transmitted by direct contact with the body fluids of infected person and objects contaminated with virus or infected animals. World Health Organization (WHO) has declared EVD epidemic as public health emergency of international concern with severe global economic burden. At fatal EBOV infection stage, patients usually die before the antibody response. Currently, rapid blood tests to diagnose EBOV infection include the antigen or antibodies capture using ELISA and RNA detection using RT/Q-PCR within 3-10 days after the onset of symptoms. Moreover, few nanotechnology-based colorimetric and paper-based immunoassay methods have been recently reported to detect Ebola virus. Unfortunately, these methods are limited to laboratory only. As state-of-the art (SoA) diagnostics time to confirm Ebola infection, varies from 6h to about 3 days, it causes delay in therapeutic approaches. Thus developing a cost-effective, rapid, sensitive, and selective sensor to detect EVD at point-of-care (POC) is certainly worth exploring to establish rapid diagnostics to decide therapeutics. This review highlights SoA of Ebola diagnostics and also a call to develop rapid, selective and sensitive POC detection of EBOV for global health care. We propose that adopting miniaturized electrochemical EBOV immunosensing can detect virus level at pM concentration within ∼40min compared to 3 days of ELISA test at nM levels.
Keywords: Ebola Virus diseases; Ebola diagnostics; Ebola sensor; Ebola therapeutics; Point-of-care sensing.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures














References
-
- Baden L.R., Kanapathipillai R., Campion E.W., Morrissey S., Rubin E.J., Drazen J.M. Ebola - an ongoing crisis. N. Engl. J. Med. 2014;371(15):1458–1459. - PubMed
-
- Bah E.I., Lamah M.-C., Fletcher T., Jacob S.T., Brett-Major D.M., Sall A.A., Shindo N., Fischer W.A., Lamontagne F., Saliou S.M., Bausch D.G., Moumié B., Jagatic T., Sprecher A., Lawler J.V., Mayet T., Jacquerioz F.A., Mendez Baggi M.F., Vallenas C., Clement C., Mardel S., Faye O., Faye O., Soropogui B., Magassouba N., Koivogui L., Pinto R., Fowler R.A. Vol. 372. 2014. Clinical Presentation of Patients with Ebola Virus Disease in Conakry, Guinea; pp. 40–47. (N. Engl. J. Med.). - PubMed
-
- Baize S., Pannetier D., Oestereich L., Rieger T., Koivogui L., Magassouba N.F., Soropogui B., Sow M.S., Keïta S., De Clerck H., Tiffany A., Dominguez G., Loua M., Traoré A., Kolié M., Malano E.R., Heleze E., Bocquin A., Mély S., Raoul H., Caro Vr, Cadar Dn, Gabriel M., Pahlmann M., Tappe D., Schmidt-Chanasit J., Impouma B., Diallo A.K., Formenty P., Van Herp M., Günther S. Emergence of Zaire Ebola Virus Disease in Guinea. N. Engl. J. Med. 2014;371(15):1418–1425. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

