Oxidative stress and ROS metabolism via down-regulation of sirtuin 3 expression in Cmah-null mice affect hearing loss
- PMID: 26319214
- PMCID: PMC4586103
- DOI: 10.18632/aging.100800
Oxidative stress and ROS metabolism via down-regulation of sirtuin 3 expression in Cmah-null mice affect hearing loss
Abstract
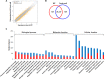
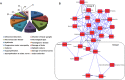
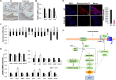
CMP-Neu5Ac hydroxylase (Cmah) disruption caused several abnormalities and diseases including hearing loss in old age. However, underling molecular mechanisms that give rise to age-related hearing loss (AHL) in Cmah-null mouse are still obscure. In this study, Cmah-null mice showed age-related decline of hearing associated with loss of sensory hair cells, spiral ganglion neurons, and/or stria vascularis degeneration in the cochlea. To identify differential gene expression profiles and pathway associated with AHL, we performed microarray analysis using Illumina MouseRef-8 v2 Expression BeadChip and pathway-focused PCR array in the cochlear tissues of Cmah-null mouse. Pathway and molecular mechanism analysis using differentially expressed genes provided evidences that altered biological pathway due to oxidative damage by low expressed antioxidants and dysregulated reactive oxygen species (ROS) metabolism. Especially, low sirtuin 3 (Sirt3) gene expressions in Cmah-null mice decreased both of downstream regulator (Foxo1 and MnSod) and regulatory transcription factor (Hif1αand Foxo3α) gene expression. Taken together, we suggest that down-regulation of Sirt3 expression leads to oxidative stress and mitochondrial dysfunction by regulation of ROS and that it could alter various signaling pathways in Cmah-null mice with AHL.
Keywords: CMP-N-acetylneuraminic acid hydroxylase; ROS metabolism; hearing loss; mitochondria dysfunction; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interests.
Figures

References
-
- Muchmore EA, Milewski M, Varki A, Diaz S. Biosynthesis of N-glycolyneuraminic acid. The primary site of hydroxylation of N-acetylneuraminic acid is the cytosolic sugar nucleotide pool. J Biol Chem. 1989;264:20216–20223. - PubMed
-
- Kozutsumi Y, Kawano T, Yamakawa T, Suzuki A. Participation of cytochrome b5 in CMP-N-acetylneuraminic acid hydroxylation in mouse liver cytosol. J Biochem. 1990;108:704–706. - PubMed
-
- Irie A, Koyama S, Kozutsumi Y, Kawasaki T, Suzuki A. The molecular basis for the absence of N-glycolylneuraminic acid in humans. J Biol Chem. 1998;273:15866–15871. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

