Vasopressin-induced stimulation of the Na(+)-activated K(+) channels is responsible for maintaining the basolateral K(+) conductance of the thick ascending limb (TAL) in EAST/SeSAME syndrome
- PMID: 26319417
- PMCID: PMC4610722
- DOI: 10.1016/j.bbadis.2015.08.023
Vasopressin-induced stimulation of the Na(+)-activated K(+) channels is responsible for maintaining the basolateral K(+) conductance of the thick ascending limb (TAL) in EAST/SeSAME syndrome
Abstract
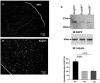
The renal phenotype of EAST syndrome, a disease caused by the loss-of-function-mutations of Kcnj10 (Kir4.1), is a reminiscence of Gitelman's syndrome characterized by the defective function in the distal convoluted tubule (DCT). The aim of the present study is to test whether antidiuretic hormone (vasopressin)-induced stimulation of the Na(+)-activated 80-150pS K(+) channel is responsible for compensating the lost function of Kcnj10 in the thick ascending limb (TAL) of subjects with EAST syndrome. Immunostaining and western blot showed that the expression of aquaporin 2 (AQP2) was significantly higher in Kcnj10(-/-) mice than those of WT littermates, suggesting that the disruption of Kcnj10 stimulates vasopressin response in the kidney. The role of vasopressin in stimulating the basolateral K(+) conductance of the TAL was strongly indicated by the finding that the application of arginine-vasopressin (AVP) hyperpolarized the membrane in the TAL of Kcnj10(-/-) mice. Application of AVP significantly stimulated the 80-150pS K(+) channel in the TAL and this effect was blocked by tolvaptan (V2 receptor antagonist) or by inhibiting PKA. Moreover, the water restriction for 24h significantly increased the probability of finding the 80-150pS K(+) channel and the K(+) channel open probability in the TAL. The application of a membrane permeable cAMP analog also mimicked the effect of AVP and activated this K(+) channel, suggesting that cAMP-PKA pathway stimulates the 80-150pS K(+) channels. The role of the basolateral K(+) conductance in maintaining transcellular Cl(-) transport is further suggested by the finding that the inhibition of basolateral K(+) channels significantly diminished the AVP-induced stimulation of the basolateral 10pS Cl(-) channels. We conclude that vasopressin stimulates the 80-150pS K(+) channel in the TAL via a cAMP-dependent mechanism. The vasopressin-induced stimulation of K(+) channels is responsible for compensating lost function of Kcnj10 thereby rescuing the basolateral K(+) conductance which is essential for the transport function in the TAL.
Keywords: ClC-kb; Kcnj10; Kcnj16; NKCC2; PKA.
Copyright © 2015. Published by Elsevier B.V.
Figures



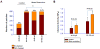
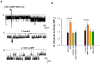


References
-
- Bandulik S, Schmidt K, Bockenhauer D, Zdebik AA, humberg E, Kleta R, Warth R, Reichold M. The salt-wasting phenotype of EAST syndrome, a disease with multifaceted symptoms linked to the KCNJ10 K channel. Pflugers Arch. 2011;461:423–435. - PubMed
-
- Bockenhauer D, Feather S, Stanescu HC, Bandulik S, Zdebik AA, Reichold M, Tobin J, Lieberer E, Sterner C, Landoure G, Arora R, Sirimanna T, Thompson D, Cross JH, van't Hoff W, Al Masri O, Tullus K, Yeung S, Anikster Y, Klootwijk E, Hubank M, Dillon MJ, Heitzmann D, Arcos-Burgos M, Knepper MA, Dobbie A, Gahl WA, Warth R, Sheridan E, Kleta R. Epilepsy Ataxia, Sensorineural Deafness, Tubulopathy, and KCNJ10 Mutations. N Engl J Med. 2009;360:1960–1970. - PMC - PubMed
-
- Gimenez I, Forbush B. Short-term stimulation of the renal Na-K-Cl cotransporter (NKCC2) by vasopressin involves phosphorylation and membrane translocation of the protein. J Biol Chem. 2003;278:26946–26951. - PubMed
-
- Greger R. Ion transport mechanisms in thick ascending limb of Henle's loop of mammalian nephron. Physiological Reviews. 1985;65:760–797. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

