Epigallocatechin-3-gallate rapidly remodels PAP85-120, SEM1(45-107), and SEM2(49-107) seminal amyloid fibrils
- PMID: 26319581
- PMCID: PMC4582112
- DOI: 10.1242/bio.010215
Epigallocatechin-3-gallate rapidly remodels PAP85-120, SEM1(45-107), and SEM2(49-107) seminal amyloid fibrils
Abstract
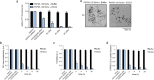
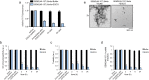
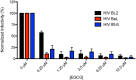
Semen harbors amyloid fibrils formed by proteolytic fragments of prostatic acid phosphatase (PAP248-286 and PAP85-120) and semenogelins (SEM1 and SEM2) that potently enhance HIV infectivity. Amyloid but not soluble forms of these peptides enhance HIV infection. Thus, agents that remodel these amyloid fibrils could prevent HIV transmission. Here, we confirm that the green tea polyphenol, epigallocatechin-3-gallate (EGCG), slowly remodels fibrils formed by PAP248-286 termed SEVI (semen derived enhancer of viral infection) and also exerts a direct anti-viral effect. We elucidate for the first time that EGCG remodels PAP85-120, SEM1(45-107), and SEM2(49-107) fibrils more rapidly than SEVI fibrils. We establish EGCG as the first small molecule that can remodel all four classes of seminal amyloid. The combined anti-amyloid and anti-viral properties of EGCG could have utility in preventing HIV transmission.
Keywords: EGCG; HIV infectivity; PAP85-120; SEM1; SEM2; SEVI.
© 2015. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures


References
-
- Arnold F., Schnell J., Zirafi O., Sturzel C., Meier C., Weil T., Standker L., Forssmann W.-G., Roan N. R., Greene W. C. et al. (2012). Naturally occurring fragments from two distinct regions of the prostatic acid phosphatase form amyloidogenic enhancers of HIV infection. J. Virol. 86, 1244-1249. 10.1128/JVI.06121-11 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

