Kinetics of drug action in disease states: towards physiology-based pharmacodynamic (PBPD) models
- PMID: 26319673
- PMCID: PMC4582079
- DOI: 10.1007/s10928-015-9437-x
Kinetics of drug action in disease states: towards physiology-based pharmacodynamic (PBPD) models
Abstract
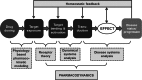
Gerhard Levy started his investigations on the "Kinetics of Drug Action in Disease States" in the fall of 1980. The objective of his research was to study inter-individual variation in pharmacodynamics. To this end, theoretical concepts and experimental approaches were introduced, which enabled assessment of the changes in pharmacodynamics per se, while excluding or accounting for the cofounding effects of concomitant changes in pharmacokinetics. These concepts were applied in several studies. The results, which were published in 45 papers in the years 1984-1994, showed considerable variation in pharmacodynamics. These initial studies on kinetics of drug action in disease states triggered further experimental research on the relations between pharmacokinetics and pharmacodynamics. Together with the concepts in Levy's earlier publications "Kinetics of Pharmacologic Effects" (Clin Pharmacol Ther 7(3): 362-372, 1966) and "Kinetics of pharmacologic effects in man: the anticoagulant action of warfarin" (Clin Pharmacol Ther 10(1): 22-35, 1969), they form a significant impulse to the development of physiology-based pharmacodynamic (PBPD) modeling as novel discipline in the pharmaceutical sciences. This paper reviews Levy's research on the "Kinetics of Drug Action in Disease States". Next it addresses the significance of his research for the evolution of PBPD modeling as a scientific discipline. PBPD models contain specific expressions to characterize in a strictly quantitative manner processes on the causal path between exposure (in terms of concentration at the target site) and the drug effect (in terms of the change in biological function). Pertinent processes on the causal path are: (1) target site distribution, (2) target binding and activation and (3) transduction and homeostatic feedback.
Keywords: Biophase distribution; Disease systems analysis; Dynamical systems analysis; Receptor theory.
Figures



References
-
- Levy G. Clinical pharmacokinetics. Washington: Academy of Pharmaceutical Sciences; 1974.
-
- Evans WES, Jerome J, Jusko WJ (1980) Applied pharmacokinetics: principles of therapeutic drug monitoring. Applied Therapeutics Inc., San Francisco, vol 3. Biopharmaceutics & Drug Disposition, Wiley. doi:10.1002/bdd.2510030315
-
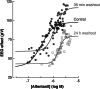
- Danhof M, Levy G. Kinetics of drug action in disease states. I. Effect of infusion rate on phenobarbital concentrations in serum, brain and cerebrospinal fluid of normal rats at onset of loss of righting reflex. J Pharmacol Exp Ther. 1984;229(1):44–50. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

