Unique plasmids generated via pUC replicon mutagenesis in an error-prone thermophile derived from Geobacillus kaustophilus HTA426
- PMID: 26319877
- PMCID: PMC4592879
- DOI: 10.1128/AEM.01574-15
Unique plasmids generated via pUC replicon mutagenesis in an error-prone thermophile derived from Geobacillus kaustophilus HTA426
Abstract
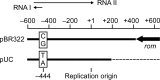
The plasmid pGKE75-catA138T, which comprises pUC18 and the catA138T gene encoding thermostable chloramphenicol acetyltransferase with an A138T amino acid replacement (CATA138T), serves as an Escherichia coli-Geobacillus kaustophilus shuttle plasmid that confers moderate chloramphenicol resistance on G. kaustophilus HTA426. The present study examined the thermoadaptation-directed mutagenesis of pGKE75-catA138T in an error-prone thermophile, generating the mutant plasmid pGKE75(αβ)-catA138T responsible for substantial chloramphenicol resistance at 65°C. pGKE75(αβ)-catA138T contained no mutation in the catA138T gene but had two mutations in the pUC replicon, even though the replicon has no apparent role in G. kaustophilus. Biochemical characterization suggested that the efficient chloramphenicol resistance conferred by pGKE75(αβ)-catA138T is attributable to increases in intracellular CATA138T and acetyl-coenzyme A following a decrease in incomplete forms of pGKE75(αβ)-catA138T. The decrease in incomplete plasmids may be due to optimization of plasmid replication by RNA species transcribed from the mutant pUC replicon, which were actually produced in G. kaustophilus. It is noteworthy that G. kaustophilus was transformed with pGKE75(αβ)-catA138T using chloramphenicol selection at 60°C. In addition, a pUC18 derivative with the two mutations propagated in E. coli at a high copy number independently of the culture temperature and high plasmid stability. Since these properties have not been observed in known plasmids, the outcomes extend the genetic toolboxes for G. kaustophilus and E. coli.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

