Vaccines, Adjuvants, and Dendritic Cell Activators--Current Status and Future Challenges
- PMID: 26320060
- PMCID: PMC4621212
- DOI: 10.1053/j.seminoncol.2015.05.006
Vaccines, Adjuvants, and Dendritic Cell Activators--Current Status and Future Challenges
Abstract
Cancer vaccines offer a low-toxicity approach to induce anticancer immune responses. They have shown promise for clinical benefit with one cancer vaccine approved in the United States for advanced prostate cancer. As other immune therapies are now clearly effective for treatment of advanced cancers of many histologies, there is renewed enthusiasm for optimizing cancer vaccines for use to prevent recurrence in early-stage cancers and/or to combine with other immune therapies for therapy of advanced cancers. Future advancements in vaccine therapy will involve the identification and selection of effective antigen formulations, optimization of adjuvants, dendritic cell (DC) activation, and combination therapies. In this summary we present the current practice, the broad collection of challenges, and the promising future directions of vaccine therapy for cancer.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

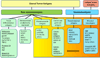
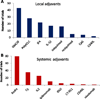

References
-
- Denkert C, von MG, Brase JC, et al. Tumor-Infiltrating Lymphocytes and Response to Neoadjuvant Chemotherapy With or Without Carboplatin in Human Epidermal Growth Factor Receptor 2-Positive and Triple-Negative Primary Breast Cancers. J Clin Oncol. 2014 JCO. - PubMed
-
- Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. - PubMed
-
- Clarke B, Tinker AV, Lee CH, et al. Intraepithelial T cells and prognosis in ovarian carcinoma: novel associations with stage, tumor type, and BRCA1 loss. Mod Pathol. 2009;22:393–402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

