Ankylosing spondylitis patients display altered dendritic cell and T cell populations that implicate pathogenic roles for the IL-23 cytokine axis and intestinal inflammation
- PMID: 26320138
- PMCID: PMC4676904
- DOI: 10.1093/rheumatology/kev245
Ankylosing spondylitis patients display altered dendritic cell and T cell populations that implicate pathogenic roles for the IL-23 cytokine axis and intestinal inflammation
Abstract
Objective: AS is a systemic inflammatory disease of the SpA family. Polymorphisms at loci including HLA-B27, IL-23R and ERAP-1 directly implicate immune mechanisms in AS pathogenesis. Previously, in an SpA model, we identified HLA-B27-mediated effects on dendritic cells that promoted disease-associated Th17 cells. Here we extend these studies to AS patients using deep immunophenotyping of candidate pathogenic cell populations. The aim of our study was to functionally characterize the immune populations mediating AS pathology.
Methods: Using 11-parameter flow cytometry, we characterized the phenotype and functions of lymphocyte and myeloid cells from peripheral blood, and the synovial phenotype of AS patients and age-matched healthy controls.
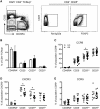
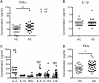
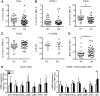
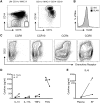
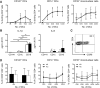
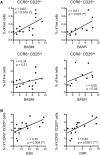
Results: Significantly fewer circulating CD1c-expressing dendritic cells were observed in AS patients, offset by an increase in CD14(-) CD16(+) mononuclear cells. Ex vivo functional analysis revealed that this latter population induced CCR6 expression and promoted secretion of IL-1β and IL-6 when co-cultured with naive CD4(+) T cells. Additionally, systemic inflammation in AS patients significantly correlated with increased proportions of activated CCR9(+) CD4(+) T cells.
Conclusion: CD14(-) CD16(+) mononuclear cells may contribute to AS by promoting Th17 responses, and antigen-presenting cells of mucosal origin are likely to contribute to systemic inflammation in AS.
Keywords: CD14− CD16+ mononuclear cells; CD4+ T cells; ankylosing spondylitis; dendritic cells; inflammation.
© The Author 2015. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures
References
-
- Baeten D, Baraliakos X, Braun J, et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet 2013;382:1705–13. - PubMed
-
- Breban M, Fernández-Sueiro JL, Richardson JA, et al. T cells, but not thymic exposure to HLA-B27, are required for the inflammatory disease of HLA-B27 transgenic rats. J Immunol 1996;156:794–803. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

