The importance of microenvironment: the role of CCL8 in metastasis formation of melanoma
- PMID: 26320180
- PMCID: PMC4745715
- DOI: 10.18632/oncotarget.5059
The importance of microenvironment: the role of CCL8 in metastasis formation of melanoma
Abstract
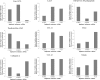
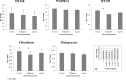
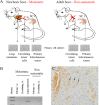
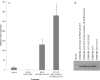
We have attempted to characterize the changes occurring on the host side during the progression of human melanoma. To investigate the role of tumor microenvironment, we set up such an animal model, which was able to isolate the host related factors playing central role in metastasis formation. One of these 'factors', CCL12, was consequently selected and its behavior was examined alongside its human homologue (CCL8). In our animal model, metastasis forming primary melanoma in the host exhibited increased level of CCL12 mRNA expression. In clinical samples, when examining the tumor and the host together, the cumulative (tumor and host) CCL8 expression was lower in the group in which human primary melanoma formed lung metastasis compared to non-metastatic primary tumors. We could not detect significant difference in CCL8 receptor (CCR1) expression between the two groups. Increased migration of the examined tumor cell lines was observed when CCL8 was applied as a chemoattractant. The tumor cells and their interactions can be influenced the expression of CCL8 by dermal fibroblasts, as a significant change in the metastatic microenvironment. Furthermore, we examined changes in miRNA profile resulted by CCL8 and miR146a appears to be a promising prognostic marker for following this process.
Keywords: CCL8; melanoma metastasis; miR146a; microenvironment.
Conflict of interest statement
There is no competing interest affecting the authors.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

