Proteomic and Biochemical Studies of Lysine Malonylation Suggest Its Malonic Aciduria-associated Regulatory Role in Mitochondrial Function and Fatty Acid Oxidation
- PMID: 26320211
- PMCID: PMC4638046
- DOI: 10.1074/mcp.M115.048850
Proteomic and Biochemical Studies of Lysine Malonylation Suggest Its Malonic Aciduria-associated Regulatory Role in Mitochondrial Function and Fatty Acid Oxidation
Abstract
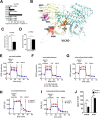
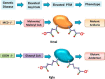
The protein substrates of sirtuin 5-regulated lysine malonylation (Kmal) remain unknown, hindering its functional analysis. In this study, we carried out proteomic screening, which identified 4042 Kmal sites on 1426 proteins in mouse liver and 4943 Kmal sites on 1822 proteins in human fibroblasts. Increased malonyl-CoA levels in malonyl-CoA decarboxylase (MCD)-deficient cells induces Kmal levels in substrate proteins. We identified 461 Kmal sites showing more than a 2-fold increase in response to MCD deficiency as well as 1452 Kmal sites detected only in MCD-/- fibroblast but not MCD+/+ cells, suggesting a pathogenic role of Kmal in MCD deficiency. Cells with increased lysine malonylation displayed impaired mitochondrial function and fatty acid oxidation, suggesting that lysine malonylation plays a role in pathophysiology of malonic aciduria. Our study establishes an association between Kmal and a genetic disease and offers a rich resource for elucidating the contribution of the Kmal pathway and malonyl-CoA to cellular physiology and human diseases.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
- Actions
- Actions
Grants and funding
- T32 AG000114/AG/NIA NIH HHS/United States
- R00CA168997/CA/NCI NIH HHS/United States
- R21 CA177925/CA/NCI NIH HHS/United States
- CA160036/CA/NCI NIH HHS/United States
- R00 CA168997/CA/NCI NIH HHS/United States
- 106169/WT_/Wellcome Trust/United Kingdom
- U24 CA160036/CA/NCI NIH HHS/United States
- R01 GM101171/GM/NIGMS NIH HHS/United States
- P30 DK020572/DK/NIDDK NIH HHS/United States
- T32-AG000114/AG/NIA NIH HHS/United States
- R01 GM105933/GM/NIGMS NIH HHS/United States
- GM105933/GM/NIGMS NIH HHS/United States
- GTB12001/TI_/Telethon/Italy
- R01GM101171/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources

