Negative Regulation of Memory Phenotype CD8 T Cell Conversion by Adhesion and Degranulation-Promoting Adapter Protein
- PMID: 26320248
- PMCID: PMC4575867
- DOI: 10.4049/jimmunol.1402670
Negative Regulation of Memory Phenotype CD8 T Cell Conversion by Adhesion and Degranulation-Promoting Adapter Protein
Abstract
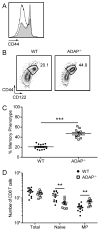
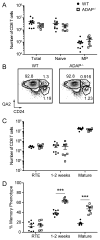
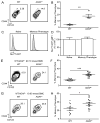
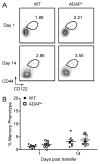
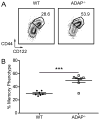
The maintenance of T cell repertoire diversity involves the entry of newly developed T cells, as well as the maintenance of memory T cells generated from previous infections. This balance depends on competition for a limited amount of homeostatic cytokines and interaction with self-peptide MHC class I. In the absence of prior infection, memory-like or memory phenotype (MP) CD8 T cells can arise from homeostatic cytokine exposure during neonatal lymphopenia. Aside from downstream cytokine signaling, little is known about the regulation of the conversion of naive CD8 T cells to MP CD8 T cells during acute lymphopenia. We have identified a novel negative regulatory role for adhesion and degranulation-promoting adapter protein (ADAP) in CD8 T cell function. We show that in the absence of ADAP, naive CD8 T cells exhibit a diminished response to stimulatory Ag, but an enhanced response to weak agonist-altered peptide ligands. ADAP-deficient mice exhibit more MP CD8 T cells that occur following thymic emigration and are largely T cell intrinsic. Naive ADAP-deficient CD8 T cells are hyperresponsive to lymphopenia in vivo and exhibit enhanced activation of STAT5 and homeostatic Ag-independent proliferation in response to IL-15. Our results indicate that ADAP dampens naive CD8 T cell responses to lymphopenia and IL-15, and they demonstrate a novel Ag-independent function for ADAP in the suppression of MP CD8 T cell generation.
Copyright © 2015 by The American Association of Immunologists, Inc.
Figures

Similar articles
-
Adhesion- and Degranulation-Promoting Adapter Protein Promotes CD8 T Cell Differentiation and Resident Memory Formation and Function during an Acute Infection.J Immunol. 2016 Sep 15;197(6):2079-89. doi: 10.4049/jimmunol.1501805. Epub 2016 Aug 12. J Immunol. 2016. PMID: 27521337 Free PMC article.
-
Defective positive selection results in T cell lymphopenia and increased autoimmune diabetes in ADAP-deficient BDC2.5-C57BL/6 mice.Eur J Immunol. 2008 Apr;38(4):986-94. doi: 10.1002/eji.200737881. Eur J Immunol. 2008. PMID: 18383041 Free PMC article.
-
Inactivation of T-cell receptor-mediated integrin activation prolongs allograft survival in ADAP-deficient mice.Transplantation. 2007 Aug 15;84(3):400-6. doi: 10.1097/01.tp.0000269724.06142.92. Transplantation. 2007. PMID: 17700167
-
Opinion: Virtual memory CD8 T cells and lymphopenia-induced memory CD8 T cells represent a single subset: Homeostatic memory T cells.Immunol Lett. 2018 Nov;203:57-61. doi: 10.1016/j.imlet.2018.09.003. Epub 2018 Sep 20. Immunol Lett. 2018. PMID: 30243945 Review.
-
Epigenetic Maintenance of Acquired Gene Expression Programs during Memory CD8 T Cell Homeostasis.Front Immunol. 2018 Jan 18;9:6. doi: 10.3389/fimmu.2018.00006. eCollection 2018. Front Immunol. 2018. PMID: 29403491 Free PMC article. Review.
Cited by
-
The Clinical Aspect of Adaptor Molecules in T Cell Signaling: Lessons Learnt From Inborn Errors of Immunity.Front Immunol. 2021 Aug 12;12:701704. doi: 10.3389/fimmu.2021.701704. eCollection 2021. Front Immunol. 2021. PMID: 34456914 Free PMC article. Review.
-
Immune Cell-Type Specific Ablation of Adapter Protein ADAP Differentially Modulates EAE.Front Immunol. 2019 Oct 1;10:2343. doi: 10.3389/fimmu.2019.02343. eCollection 2019. Front Immunol. 2019. PMID: 31632410 Free PMC article.
-
Adhesion- and Degranulation-Promoting Adapter Protein Promotes CD8 T Cell Differentiation and Resident Memory Formation and Function during an Acute Infection.J Immunol. 2016 Sep 15;197(6):2079-89. doi: 10.4049/jimmunol.1501805. Epub 2016 Aug 12. J Immunol. 2016. PMID: 27521337 Free PMC article.
-
DOCK2 Sets the Threshold for Entry into the Virtual Memory CD8+ T Cell Compartment by Negatively Regulating Tonic TCR Triggering.J Immunol. 2020 Jan 1;204(1):49-57. doi: 10.4049/jimmunol.1900440. Epub 2019 Nov 18. J Immunol. 2020. PMID: 31740487 Free PMC article.
References
-
- Takada K, Jameson SC. Naive T cell homeostasis: from awareness of space to a sense of place. Nat Rev Immunol. 2009;9:823–832. - PubMed
-
- Min B, McHugh R, Sempowski GD, Mackall C, Foucras G, Paul WE. Neonates support lymphopenia-induced proliferation. Immunity. 2003;18:131–140. - PubMed
-
- Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

