Intrinsic Differences in Donor CD4 T Cell IL-2 Production Influence Severity of Parent-into-F1 Murine Lupus by Skewing the Immune Response Either toward Help for B Cells and a Sustained Autoantibody Response or toward Help for CD8 T Cells and a Downregulatory Th1 Response
- PMID: 26320249
- PMCID: PMC4575913
- DOI: 10.4049/jimmunol.1402782
Intrinsic Differences in Donor CD4 T Cell IL-2 Production Influence Severity of Parent-into-F1 Murine Lupus by Skewing the Immune Response Either toward Help for B Cells and a Sustained Autoantibody Response or toward Help for CD8 T Cells and a Downregulatory Th1 Response
Abstract
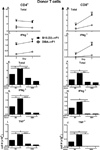
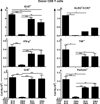
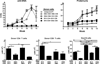
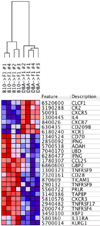
Using the parent-into-F1 model of induced lupus and (C57BL/6 × DBA2) F1 mice as hosts, we compared the inherent lupus-inducing properties of the two parental strain CD4 T cells. To control for donor CD4 recognition of alloantigen, we used H-2(d) identical DBA/2 and B10.D2 donor T cells. We demonstrate that these two normal, nonlupus-prone parental strains exhibit two different T cell activation pathways in vivo. B10.D2 CD4 T cells induce a strong Th1/CMI pathway that is characterized by IL-2/IFN-γ expression, help for CD8 CTLs, and skewing of dendritic cell (DC) subsets toward CD8a DCs, coupled with reduced CD4 T follicular helper cells and transient B cell help. In contrast, DBA/2 CD4 T cells exhibit a reciprocal, lupus-inducing pathway that is characterized by poor IL-2/IFN-γ expression, poor help for CD8 CTLs, and skewing of DC subsets toward plasmacytoid DCs, coupled with greater CD4 T follicular helper cells, prolonged B cell activation, autoantibody formation, and lupus-like renal disease. Additionally, two distinct in vivo splenic gene-expression signatures were induced. In vitro analysis of TCR signaling revealed defective DBA CD4 T cell induction of NF-κB, reduced degradation of IκBα, and increased expression of the NF-κB regulator A20. Thus, attenuated NF-κB signaling may lead to diminished IL-2 production by DBA CD4 T cells. These results indicate that intrinsic differences in donor CD4 IL-2 production and subsequent immune skewing could contribute to lupus susceptibility in humans. Therapeutic efforts to skew immune function away from excessive help for B cells and toward help for CTLs may be beneficial.
Copyright © 2015 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures







Similar articles
-
CTL-Promoting Effects of IL-21 Counteract Murine Lupus in the Parent→F1 Graft-versus-Host Disease Model.J Immunol. 2016 Feb 15;196(4):1529-40. doi: 10.4049/jimmunol.1501824. Epub 2016 Jan 20. J Immunol. 2016. PMID: 26792801
-
Kinetics of Th1 and Th2 cytokine production during the early course of acute and chronic murine graft-versus-host disease. Regulatory role of donor CD8+ T cells.J Immunol. 1995 Sep 1;155(5):2396-406. J Immunol. 1995. PMID: 7650373
-
The parent-into-F1 murine model in the study of lupus-like autoimmunity and CD8 cytotoxic T lymphocyte function.Methods Mol Biol. 2012;900:253-70. doi: 10.1007/978-1-60761-720-4_12. Methods Mol Biol. 2012. PMID: 22933073
-
T cells, murine chronic graft-versus-host disease and autoimmunity.J Autoimmun. 2012 Sep;39(3):240-7. doi: 10.1016/j.jaut.2012.05.017. Epub 2012 Jun 16. J Autoimmun. 2012. PMID: 22704961 Free PMC article. Review.
-
B cell contribution of the CD4+ T cell inflammatory phenotypes in systemic lupus erythematosus.Autoimmunity. 2017 Feb;50(1):37-41. doi: 10.1080/08916934.2017.1280028. Autoimmunity. 2017. PMID: 28166683 Free PMC article. Review.
Cited by
-
Hybrid cytokine IL233 renders protection in murine acute graft vs host disease (aGVHD).Cell Immunol. 2021 Jun;364:104345. doi: 10.1016/j.cellimm.2021.104345. Epub 2021 Mar 23. Cell Immunol. 2021. PMID: 33831754 Free PMC article.
-
Sex differences in donor T cell targeting of host splenocyte subpopulations in acute and chronic murine graft-vs.-host disease: implications for lupus-like autoimmunity.bioRxiv [Preprint]. 2024 Jun 10:2024.06.07.595177. doi: 10.1101/2024.06.07.595177. bioRxiv. 2024. PMID: 38915570 Free PMC article. Preprint.
-
In vivo IL-4 prevents allo-antigen driven CD8+ CTL development.Clin Immunol. 2017 Jul;180:11-24. doi: 10.1016/j.clim.2017.03.008. Epub 2017 Mar 27. Clin Immunol. 2017. PMID: 28359782 Free PMC article.
-
A MLR-Based Approach to Analyze Regulators of T Lymphocyte Activation In Vivo.Int J Mol Sci. 2022 May 10;23(10):5337. doi: 10.3390/ijms23105337. Int J Mol Sci. 2022. PMID: 35628145 Free PMC article.
-
New Mechanisms and Therapeutic Targets in Systemic Lupus Erythematosus.MedComm (2020). 2025 Jun 9;6(6):e70246. doi: 10.1002/mco2.70246. eCollection 2025 Jun. MedComm (2020). 2025. PMID: 40491969 Free PMC article. Review.
References
-
- Shlomchik MJ, Marshak-Rothstein A, Wolfowicz CB, Rothstein TL, Weigert MG. The role of clonal selection and somatic mutation in autoimmunity. Nature. 1987;328:805–811. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

