Not All SCID Pigs Are Created Equally: Two Independent Mutations in the Artemis Gene Cause SCID in Pigs
- PMID: 26320255
- PMCID: PMC5621739
- DOI: 10.4049/jimmunol.1501132
Not All SCID Pigs Are Created Equally: Two Independent Mutations in the Artemis Gene Cause SCID in Pigs
Abstract
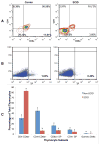
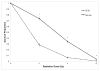
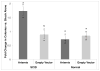
Mutations in >30 genes are known to result in impairment of the adaptive immune system, causing a group of disorders collectively known as SCID. SCID disorders are split into groups based on their presence and/or functionality of B, T, and NK cells. Piglets from a line of Yorkshire pigs at Iowa State University were shown to be affected by T(-)B(-)NK(+) SCID, representing, to our knowledge, the first example of naturally occurring SCID in pigs. In this study, we present evidence for two spontaneous mutations as the molecular basis for this SCID phenotype. Flow cytometry analysis of thymocytes showed an increased frequency of immature T cells in SCID pigs. Fibroblasts from these pigs were more sensitive to ionizing radiation than non-SCID piglets, eliminating the RAG1 and RAG2 genes. Genetic and molecular analyses showed that two mutations were present in the Artemis gene, which in the homozygous or compound heterozygous state cause the immunodeficient phenotype. Rescue of SCID fibroblast radiosensitivity by human Artemis protein demonstrated that the identified Artemis mutations are the direct cause of this cellular phenotype. The work presented in the present study reveals two mutations in the Artemis gene that cause T(-)B(-)NK(+) SCID in pigs. The SCID pig can be an important biomedical model, but these mutations would be undesirable in commercial pig populations. The identified mutations and associated genetic tests can be used to address both of these issues.
Copyright © 2015 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors have filed a patent that includes testing for the genetic mutations discovered in this study.
Figures



Similar articles
-
NK cells are intrinsically functional in pigs with Severe Combined Immunodeficiency (SCID) caused by spontaneous mutations in the Artemis gene.Vet Immunol Immunopathol. 2016 Jul;175:1-6. doi: 10.1016/j.vetimm.2016.04.008. Epub 2016 Apr 19. Vet Immunol Immunopathol. 2016. PMID: 27269786 Free PMC article.
-
Artemis splice defects cause atypical SCID and can be restored in vitro by an antisense oligonucleotide.Genes Immun. 2011 Sep;12(6):434-44. doi: 10.1038/gene.2011.16. Epub 2011 Mar 10. Genes Immun. 2011. PMID: 21390052
-
Lentivirus Mediated Correction of Artemis-Deficient Severe Combined Immunodeficiency.Hum Gene Ther. 2017 Jan;28(1):112-124. doi: 10.1089/hum.2016.064. Epub 2016 Sep 7. Hum Gene Ther. 2017. PMID: 27611239 Free PMC article.
-
DNA-PKcs deficiency in human: long predicted, finally found.Curr Opin Allergy Clin Immunol. 2009 Dec;9(6):503-9. doi: 10.1097/ACI.0b013e3283327e41. Curr Opin Allergy Clin Immunol. 2009. PMID: 19823081 Review.
-
Mutations in genes required for T-cell development: IL7R, CD45, IL2RG, JAK3, RAG1, RAG2, ARTEMIS, and ADA and severe combined immunodeficiency: HuGE review.Genet Med. 2004 Jan-Feb;6(1):16-26. doi: 10.1097/01.GIM.0000105752.80592.A3. Genet Med. 2004. PMID: 14726805 Review.
Cited by
-
EFFECT OF GENOTYPE AND AGE ON A DEFINED MICROBIOTA IN GNOTOBIOTIC SCID PIGLETS.bioRxiv [Preprint]. 2024 Sep 7:2024.09.03.611011. doi: 10.1101/2024.09.03.611011. bioRxiv. 2024. PMID: 39282343 Free PMC article. Preprint.
-
Creating effective biocontainment facilities and maintenance protocols for raising specific pathogen-free, severe combined immunodeficient (SCID) pigs.Lab Anim. 2018 Aug;52(4):402-412. doi: 10.1177/0023677217750691. Epub 2018 Jan 11. Lab Anim. 2018. PMID: 29325489 Free PMC article.
-
Increased and prolonged human norovirus infection in RAG2/IL2RG deficient gnotobiotic pigs with severe combined immunodeficiency.Sci Rep. 2016 Apr 27;6:25222. doi: 10.1038/srep25222. Sci Rep. 2016. PMID: 27118081 Free PMC article.
-
Compared to other NHEJ factors, DNA-PK protein and RNA levels are markedly increased in all higher primates, but not in prosimians or other mammals.DNA Repair (Amst). 2024 Oct;142:103737. doi: 10.1016/j.dnarep.2024.103737. Epub 2024 Aug 8. DNA Repair (Amst). 2024. PMID: 39128395
-
Porcine signal regulatory protein alpha binds to human CD47 to inhibit phagocytosis: Implications for human hematopoietic stem cell transplantation into severe combined immunodeficient pigs.Xenotransplantation. 2019 Mar;26(2):e12466. doi: 10.1111/xen.12466. Epub 2018 Oct 12. Xenotransplantation. 2019. PMID: 30311702 Free PMC article.
References
-
- Notarangelo LD. Primary immunodeficiencies. J Allergy Clin Immunol. 2010;125:S182–S194. - PubMed
-
- Schwarz K, Gauss GH, Ludwig L, Pannicke U, Li Z, Lindner D, Friedrich W, Seger Ra, Hansen-Hagge TE, Desiderio S, Lieber MR, Bartram CR. RAG mutations in human B cell-negative SCID. Science. 1996;274:97–99. - PubMed
-
- De Villartay JP. V(D)J recombination deficiencies. Adv Exp Med Biol. 2009;650:46–58. - PubMed
-
- Blum B, Benvenisty N. The Tumorigenicity of Human Embryonic Stem Cells. Adv Cancer Res. 2008;100:133–158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

