Diphenhydramine as Adjuvant Therapy for Acute Migraine: An Emergency Department-Based Randomized Clinical Trial
- PMID: 26320523
- PMCID: PMC4695376
- DOI: 10.1016/j.annemergmed.2015.07.495
Diphenhydramine as Adjuvant Therapy for Acute Migraine: An Emergency Department-Based Randomized Clinical Trial
Abstract
Study objective: More than 1 million patients present to US emergency departments (EDs) annually seeking care for acute migraine. Parenteral antihistamines have long been used in combination with antidopaminergics such as metoclopramide to treat acute migraine in the ED. High-quality data supporting this practice do not exist. We determine whether administration of diphenhydramine 50 mg intravenously+metoclopramide 10 mg intravenously results in greater rates of sustained headache relief than placebo+metoclopramide 10 mg intravenously.
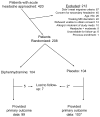
Methods: This was a randomized, double-blind, clinical trial comparing 2 active treatments for acute migraine in an ED. Eligible patients were adults younger than 65 years presenting with an acute moderate or severe headache meeting International Classification of Headache Disorders-2 migraine criteria. Patients were stratified according to presence or absence of allergic symptoms. The primary outcome was sustained headache relief, defined as achieving a headache level of mild or none within 2 hours of medication administration and maintaining this level of relief without use of any additional headache medication for 48 hours. Secondary efficacy outcomes included mean improvement on a 0 to 10 verbal scale between baseline and 1 hour, the frequency with which subjects indicated they would want the same medication the next time they present to the ED with migraine, and the ED throughput time. Sample size calculation using a 2-sided α of .05, a β of .20, and a 15% difference between study arms determined the need for 374 patients. An interim analysis was conducted when data were available for 200 subjects.
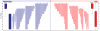
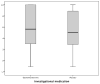
Results: Four hundred twenty patients were approached for participation. Two hundred eight eligible patients consented to participate and were randomized. At the planned interim analysis, the data and safety monitoring board recommended that the study be halted for futility. Baseline characteristics were comparable between the groups. Fourteen percent (29/208) of the sample reported allergic symptoms. Of patients randomized to diphenhydramine, 40% (40/100) reported sustained relief at 48 hours, as did 37% (38/103) of patients randomized to placebo (95% confidence interval [CI] for difference of 3%: -10% to 16%). One hour after medication administration, patients randomized to diphenhydramine improved by a mean of 5.1 on the 0 to 10 scale versus 4.8 for those randomized to placebo (95% CI for difference of 0.3: -0.6 to 1.1). Eighty-five percent (84/99) of the patients in the diphenhydramine arm reported they would want the same medication combination during a subsequent ED visit, as did 76% (77/102) of those who received placebo (95% CI for difference of 9%: -2% to 20%). Median ED length of stay was 122 minutes (interquartile range 84 to 180 minutes) in the diphenhydramine group and 139 minutes (interquartile range 90 to 235 minutes) in the placebo arm. Rates of adverse effects, including akathisia, were comparable between the groups.
Conclusion: Intravenous diphenhydramine, when administered as adjuvant therapy with metoclopramide, does not improve migraine outcomes.
Trial registration: ClinicalTrials.gov NCT01825941.
Copyright © 2016 American College of Emergency Physicians. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
We have no conflicts of interest to report.
Figures
Similar articles
-
A randomized controlled trial of prochlorperazine versus metoclopramide for treatment of acute migraine.Ann Emerg Med. 2008 Oct;52(4):399-406. doi: 10.1016/j.annemergmed.2007.09.027. Epub 2007 Nov 19. Ann Emerg Med. 2008. PMID: 18006188 Clinical Trial.
-
A randomized trial of intravenous ketorolac versus intravenous metoclopramide plus diphenhydramine for tension-type and all nonmigraine, noncluster recurrent headaches.Ann Emerg Med. 2013 Oct;62(4):311-318.e4. doi: 10.1016/j.annemergmed.2013.03.017. Epub 2013 Apr 6. Ann Emerg Med. 2013. PMID: 23567060 Free PMC article. Clinical Trial.
-
An exploratory study of IV metoclopramide+diphenhydramine for acute post-traumatic headache.Am J Emerg Med. 2018 Feb;36(2):285-289. doi: 10.1016/j.ajem.2017.10.034. Epub 2017 Oct 13. Am J Emerg Med. 2018. PMID: 29074068 Free PMC article. Clinical Trial.
-
Rescue therapy for acute migraine, part 3: opioids, NSAIDs, steroids, and post-discharge medications.Headache. 2012 Mar;52(3):467-82. doi: 10.1111/j.1526-4610.2012.02097.x. Headache. 2012. PMID: 22404708 Review.
-
The Efficacy and Safety of Prochlorperazine in Patients With Acute Migraine: A Systematic Review and Meta-Analysis.Headache. 2019 May;59(5):682-700. doi: 10.1111/head.13527. Epub 2019 Apr 16. Headache. 2019. PMID: 30990883
Cited by
-
Treatment of headache reduces blood pressure among most patients with migraine and elevated blood pressure.Am J Emerg Med. 2025 May;91:55-58. doi: 10.1016/j.ajem.2025.02.017. Epub 2025 Feb 19. Am J Emerg Med. 2025. PMID: 39987628
-
Randomized Study of Metoclopramide Plus Diphenhydramine for Acute Posttraumatic Headache.Neurology. 2021 May 4;96(18):e2323-e2331. doi: 10.1212/WNL.0000000000011822. Epub 2021 Mar 24. Neurology. 2021. PMID: 33762421 Free PMC article. Clinical Trial.
-
Prophylactic antiemetics for adults receiving intravenous opioids in the acute care setting.Cochrane Database Syst Rev. 2022 May 19;5(5):CD013860. doi: 10.1002/14651858.CD013860.pub2. Cochrane Database Syst Rev. 2022. PMID: 35588093 Free PMC article.
-
Efficacy of adding midazolam to paracetamol in pain control of patients with a primary headache: A randomized, clinical trial study.Turk J Emerg Med. 2020 May 27;20(2):63-68. doi: 10.4103/2452-2473.285011. eCollection 2020 Apr-Jun. Turk J Emerg Med. 2020. PMID: 32587924 Free PMC article.
-
Trends in the Management of Headache Disorders in US Emergency Departments: Analysis of 2007-2018 National Hospital Ambulatory Medical Care Survey Data.J Clin Med. 2022 Mar 3;11(5):1401. doi: 10.3390/jcm11051401. J Clin Med. 2022. PMID: 35268492 Free PMC article.
References
-
- Friedman BW, West J, Vinson DR, Minen MT, Restivo A, Gallagher EJ. Current management of migraine in US emergency departments: An analysis of the National Hospital Ambulatory Medical Care Survey. Cephalalgia. 2014 - PubMed
-
- Gazerani P, Pourpak Z, Ahmadiani A, Hemmati A, Kazemnejad A. A Correlation between Migraine, Histamine and Immunoglobulin E. Iran J Allergy Asthma Immunol. 2003;2:17–24. - PubMed
-
- Aamodt AH, Stovner LJ, Langhammer A, Hagen K, Zwart JA. Is headache related to asthma, hay fever, and chronic bronchitis? The Head-HUNT Study. Headache. 2007;47:204–12. - PubMed
-
- Ku M, Silverman B, Prifti N, Ying W, Persaud Y, Schneider A. Prevalence of migraine headaches in patients with allergic rhinitis. Ann Allergy Asthma Immunol. 2006;97:226–30. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

