Animal models of pituitary neoplasia
- PMID: 26320859
- PMCID: PMC4721536
- DOI: 10.1016/j.mce.2015.08.024
Animal models of pituitary neoplasia
Abstract
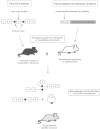
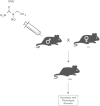
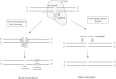
Pituitary neoplasias can occur as part of a complex inherited disorder, or more commonly as sporadic (non-familial) disease. Studies of the molecular and genetic mechanisms causing such pituitary tumours have identified dysregulation of >35 genes, with many revealed by studies in mice, rats and zebrafish. Strategies used to generate these animal models have included gene knockout, gene knockin and transgenic over-expression, as well as chemical mutagenesis and drug induction. These animal models provide an important resource for investigation of tissue-specific tumourigenic mechanisms, and evaluations of novel therapies, illustrated by studies into multiple endocrine neoplasia type 1 (MEN1), a hereditary syndrome in which ∼ 30% of patients develop pituitary adenomas. This review describes animal models of pituitary neoplasia that have been generated, together with some recent advances in gene editing technologies, and an illustration of the use of the Men1 mouse as a pre clinical model for evaluating novel therapies.
Keywords: Adenoma; Carcinoma; Mouse; Multiple endocrine neoplasia type 1; Pituitary; Rat.
Copyright © 2015 The Authors. Published by Elsevier Ireland Ltd.. All rights reserved.
Figures

Similar articles
-
Pituitary tumors and hyperplasia in multiple endocrine neoplasia type 1 syndrome (MEN1): a case-control study in a series of 77 patients versus 2509 non-MEN1 patients.Am J Surg Pathol. 2008 Apr;32(4):534-43. doi: 10.1097/PAS.0b013e31815ade45. Am J Surg Pathol. 2008. PMID: 18300794
-
Multiple endocrine neoplasia type 1.Orphanet J Rare Dis. 2006 Oct 2;1:38. doi: 10.1186/1750-1172-1-38. Orphanet J Rare Dis. 2006. PMID: 17014705 Free PMC article. Review.
-
Genetic, molecular and clinical features of familial isolated pituitary adenomas.Horm Res. 2009 Apr;71 Suppl 2:116-22. doi: 10.1159/000192448. Epub 2009 Apr 29. Horm Res. 2009. PMID: 19407507 Review.
-
Genetic mutations in sporadic pituitary adenomas--what to screen for?Nat Rev Endocrinol. 2015 Jan;11(1):43-54. doi: 10.1038/nrendo.2014.181. Epub 2014 Oct 28. Nat Rev Endocrinol. 2015. PMID: 25350067 Review.
-
The clinical, pathological, and genetic features of familial isolated pituitary adenomas.Eur J Endocrinol. 2007 Oct;157(4):371-82. doi: 10.1530/EJE-07-0348. Eur J Endocrinol. 2007. PMID: 17893250 Review.
Cited by
-
Patient-derived xenograft in zebrafish embryos: a new platform for translational research in neuroendocrine tumors.Endocrine. 2017 Aug;57(2):214-219. doi: 10.1007/s12020-016-1048-9. Epub 2016 Aug 2. Endocrine. 2017. PMID: 27481363
-
Intranasal 15d-PGJ2 inhibits the growth of rat lactotroph pituitary neuroendocrine tumors by inducing PPARγ-dependent apoptotic and autophagic cell death.Front Neurosci. 2023 May 12;17:1109675. doi: 10.3389/fnins.2023.1109675. eCollection 2023. Front Neurosci. 2023. PMID: 37250410 Free PMC article.
-
Sprouting Angiogenesis in Human Pituitary Adenomas.Front Oncol. 2022 May 5;12:875219. doi: 10.3389/fonc.2022.875219. eCollection 2022. Front Oncol. 2022. PMID: 35600354 Free PMC article.
-
The Progress of Immunotherapy in Refractory Pituitary Adenomas and Pituitary Carcinomas.Front Endocrinol (Lausanne). 2020 Dec 11;11:608422. doi: 10.3389/fendo.2020.608422. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33362722 Free PMC article. Review.
-
mTOR promotes pituitary tumor development through activation of PTTG1.Oncogene. 2017 Feb 16;36(7):979-988. doi: 10.1038/onc.2016.264. Epub 2016 Aug 15. Oncogene. 2017. PMID: 27524416
References
-
- Abbud R.A., Takumi I., Barker E.M., Ren S.G., Chen D.Y., Wawrowsky K., Melmed S. Early multipotential pituitary focal hyperplasia in the alpha-subunit of glycoprotein hormone-driven pituitary tumor-transforming gene transgenic mice. Mol. Endocrinol. 2005;19:1383–1391. - PubMed
-
- Acevedo-Arozena A., Wells S., Potter P., Kelly M., Cox R.D., Brown S.D. ENU mutagenesis, a way forward to understand gene function. Annu. Rev. Genomics Hum. Genet. 2008;9:49–69. - PubMed
-
- Angotzi A.R., Mungpakdee S., Stefansson S., Male R., Chourrout D. Involvement of Prop1 homeobox gene in the early development of fish pituitary gland. Gen. Comp. Endocrinol. 2011;171:332–340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

