Analytical Validation of Quantitative Real-Time PCR Methods for Quantification of Trypanosoma cruzi DNA in Blood Samples from Chagas Disease Patients
- PMID: 26320872
- PMCID: PMC4698797
- DOI: 10.1016/j.jmoldx.2015.04.010
Analytical Validation of Quantitative Real-Time PCR Methods for Quantification of Trypanosoma cruzi DNA in Blood Samples from Chagas Disease Patients
Abstract
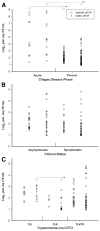
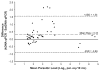
An international study was performed by 26 experienced PCR laboratories from 14 countries to assess the performance of duplex quantitative real-time PCR (qPCR) strategies on the basis of TaqMan probes for detection and quantification of parasitic loads in peripheral blood samples from Chagas disease patients. Two methods were studied: Satellite DNA (SatDNA) qPCR and kinetoplastid DNA (kDNA) qPCR. Both methods included an internal amplification control. Reportable range, analytical sensitivity, limits of detection and quantification, and precision were estimated according to international guidelines. In addition, inclusivity and exclusivity were estimated with DNA from stocks representing the different Trypanosoma cruzi discrete typing units and Trypanosoma rangeli and Leishmania spp. Both methods were challenged against 156 blood samples provided by the participant laboratories, including samples from acute and chronic patients with varied clinical findings, infected by oral route or vectorial transmission. kDNA qPCR showed better analytical sensitivity than SatDNA qPCR with limits of detection of 0.23 and 0.70 parasite equivalents/mL, respectively. Analyses of clinical samples revealed a high concordance in terms of sensitivity and parasitic loads determined by both SatDNA and kDNA qPCRs. This effort is a major step toward international validation of qPCR methods for the quantification of T. cruzi DNA in human blood samples, aiming to provide an accurate surrogate biomarker for diagnosis and treatment monitoring for patients with Chagas disease.
Copyright © 2015 American Society for Investigative Pathology and the Association for Molecular Pathology. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Parasitemia Levels in Trypanosoma cruzi Infection in Spain, an Area Where the Disease Is Not Endemic: Trends by Different Molecular Approaches.Microbiol Spectr. 2022 Oct 26;10(5):e0262822. doi: 10.1128/spectrum.02628-22. Epub 2022 Oct 3. Microbiol Spectr. 2022. PMID: 36190410 Free PMC article.
-
Mitochondrial and satellite real time-PCR for detecting T. cruzi DTU II strain in blood and organs of experimentally infected mice presenting different levels of parasite load.Exp Parasitol. 2019 May;200:13-15. doi: 10.1016/j.exppara.2019.03.007. Epub 2019 Mar 20. Exp Parasitol. 2019. PMID: 30904696 Review.
-
International study to evaluate PCR methods for detection of Trypanosoma cruzi DNA in blood samples from Chagas disease patients.PLoS Negl Trop Dis. 2011 Jan 11;5(1):e931. doi: 10.1371/journal.pntd.0000931. PLoS Negl Trop Dis. 2011. PMID: 21264349 Free PMC article.
-
Identification of Trypanosoma cruzi Discrete Typing Units (DTUs) in Latin-American migrants in Barcelona (Spain).Parasitol Int. 2017 Apr;66(2):83-88. doi: 10.1016/j.parint.2016.12.003. Epub 2016 Dec 7. Parasitol Int. 2017. PMID: 27940065
-
The use of qPCR in human Chagas disease: a systematic review.Expert Rev Mol Diagn. 2019 Oct;19(10):875-894. doi: 10.1080/14737159.2019.1659729. Epub 2019 Sep 7. Expert Rev Mol Diagn. 2019. PMID: 31496309
Cited by
-
Worldwide Control and Management of Chagas Disease in a New Era of Globalization: a Close Look at Congenital Trypanosoma cruzi Infection.Clin Microbiol Rev. 2022 Apr 20;35(2):e0015221. doi: 10.1128/cmr.00152-21. Epub 2022 Mar 3. Clin Microbiol Rev. 2022. PMID: 35239422 Free PMC article. Review.
-
Improved DNA extraction technique from clot for the diagnosis of Chagas disease.PLoS Negl Trop Dis. 2019 Jan 11;13(1):e0007024. doi: 10.1371/journal.pntd.0007024. eCollection 2019 Jan. PLoS Negl Trop Dis. 2019. PMID: 30633743 Free PMC article.
-
Monitoring the parasite load in chronic Chagas disease patients: comparison between blood culture and quantitative real time PCR.PLoS One. 2018 Nov 29;13(11):e0208133. doi: 10.1371/journal.pone.0208133. eCollection 2018. PLoS One. 2018. PMID: 30496249 Free PMC article.
-
Trypanosoma cruzi load in synanthropic rodents from rural areas in Chile.Parasit Vectors. 2018 Mar 12;11(1):171. doi: 10.1186/s13071-018-2771-2. Parasit Vectors. 2018. PMID: 29530074 Free PMC article.
-
First external quality assurance program for bloodstream Real-Time PCR monitoring of treatment response in clinical trials of Chagas disease.PLoS One. 2017 Nov 27;12(11):e0188550. doi: 10.1371/journal.pone.0188550. eCollection 2017. PLoS One. 2017. PMID: 29176887 Free PMC article.
References
-
- Organización Panamericana de la Salud (OPS) Estimación cuantitativa de la enfermedad de Chagas en las Américas. Montevideo, Uruguay: OPS; 2006. OPS/HDM/CD/425-06.
-
- Schmunis GA, Yadon ZE. Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop. 2010;115:14–21. - PubMed
-
- Prata A. Clinical and epidemiological aspects of Chagas disease. Lancet Infect Dis. 2001;1:92–100. - PubMed
-
- WHO/TDR Disease Reference Group on Chagas Disease, Human African Trypanosomiasis and Leishmaniasis. Research Priorities for Chagas Disease, Human African Trypanosomiasis and Leishmaniasis. Geneva, Switzerland: WHO; 2012. WHO Technical Report Series (No. 975) - PubMed
-
- Zingales B, Miles MA, Campbell DA, Tibayrenc M, Macedo AM, Teixeira MM, Schijman AG, Llewellyn MS, Lages-Silva E, Machado CR, Andrade SG, Sturm NR. The revised Trypanosoma cruzi subspecific nomenclature: rationale, epidemiological relevance and research applications. Infect Genet Evol. 2012;12:240–253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

