CEND1 and NEUROGENIN2 Reprogram Mouse Astrocytes and Embryonic Fibroblasts to Induced Neural Precursors and Differentiated Neurons
- PMID: 26321141
- PMCID: PMC4618597
- DOI: 10.1016/j.stemcr.2015.07.012
CEND1 and NEUROGENIN2 Reprogram Mouse Astrocytes and Embryonic Fibroblasts to Induced Neural Precursors and Differentiated Neurons
Abstract
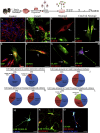
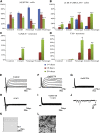
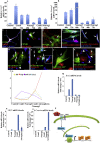
Recent studies demonstrate that astroglia from non-neurogenic brain regions can be reprogrammed into functional neurons through forced expression of neurogenic factors. Here we explored the effect of CEND1 and NEUROG2 on reprogramming of mouse cortical astrocytes and embryonic fibroblasts. Forced expression of CEND1, NEUROG2, or both resulted in acquisition of induced neuronal cells expressing subtype-specific markers, while long-term live-cell imaging highlighted the existence of two different modes of neuronal trans-differentiation. Of note, a subpopulation of CEND1 and NEUROG2 double-transduced astrocytes formed spheres exhibiting neural stem cell properties. mRNA and protein expression studies revealed a reciprocal feedback loop existing between the two molecules, while knockdown of endogenous CEND1 demonstrated that it is a key mediator of NEUROG2-driven neuronal reprogramming. Our data suggest that common reprogramming mechanisms exist driving the conversion of lineage-distant somatic cell types to neurons and reveal a critical role for CEND1 in NEUROG2-driven astrocytic reprogramming.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Caiazzo M., Dell’Anno M.T., Dvoretskova E., Lazarevic D., Taverna S., Leo D., Sotnikova T.D., Menegon A., Roncaglia P., Colciago G. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476:224–227. - PubMed
-
- Costa M.R., Ortega F., Brill M.S., Beckervordersandforth R., Petrone C., Schroeder T., Götz M., Berninger B. Continuous live imaging of adult neural stem cell division and lineage progression in vitro. Development. 2011;138:1057–1068. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

