Neoadjuvant Rectal (NAR) Score: a New Surrogate Endpoint in Rectal Cancer Clinical Trials
- PMID: 26321890
- PMCID: PMC4550644
- DOI: 10.1007/s11888-015-0285-2
Neoadjuvant Rectal (NAR) Score: a New Surrogate Endpoint in Rectal Cancer Clinical Trials
Abstract
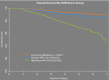
The conduct of clinical trials in colorectal cancer has historically relied upon endpoints such as disease-free (DFS) or overall survival (OS). While ideal, these endpoints require long-term follow-up, thus contributing to a slow pace of scientific progress in clinical research. Identification of short-term endpoints to serve as surrogates for DFS and OS would enable more rapid determination of success or failure of an experimental intervention and thus facilitate more scientific discovery and progress leading to clinical practice improvements. In rectal cancer clinical trials, there have been few validated alternatives to DFS and OS, including pathologic complete response (ypCR). The neoadjuvant rectal (NAR) score was developed as a composite short-term endpoint for clinical trials involving neoadjuvant therapy for rectal cancer. The NAR score is based upon variables routinely collected and available to clinical investigators during the conduct of prospective studies. Based upon two independent validation datasets, the NAR score predicts OS in rectal cancer clinical trials better than ypCR. While final dataset validation is ongoing, the NAR score offers an opportunity to incorporate a novel surrogate endpoint into early phase rectal cancer clinical trials.
Keywords: Clinical trial; Endpoint; NAR score; Neoadjuvant; Nomogram; Pathologic complete response; Rectal cancer; Surrogate; pCR.
Figures
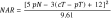
References
-
- Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2012, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2012/, based on November 2014 SEER data submission, posted to the SEER web site, April 2015.
-
- American Cancer Society . Cancer facts & figures 2015. Atlanta: American Cancer Society; 2015.
-
- NCCN Clinical Practice Guidelines in Oncology™ (Rectal Cancer). V.3.2015. Accessed from the National Comprehensive Cancer Network website <http://www.nccn.org/professionals/physician_gls/PDF/rectal.pdf> on June 20, 2015.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

